Table A.3
Uptake coefficients for heterogeneous reactions involving chlorinated species.
| Label | Reaction | Uptake coefficient | Reference |
|---|---|---|---|
| γ1 | 6 H2O + dust → Cl + 6 H2O | 0.1 ×6.3 × 10−2 × jClO(z) | [3] based on [20] and [21] |
| γ2 | 2 HCl + dust → 2H | MPM(0.2, 2.03×103, 12.30×103) | [3] based on [6] and [7] |
| γ3 | HCl + water ice → H + Cl or H | MPM(0.09, 8.33×108, 30.50×103) × (1 - θLang) | [3] based on [8] and [9] |
| γ4 | Cl2 + dust → Ø | 1×10−3 | [2] (upper limit) |
| γ5 | ClO + water ice → O | 1×10−4 | [2] (upper limit) |
Notes. The MPM function is MPM(A, 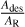 , ΔE) = A/[1 +
, ΔE) = A/[1 + 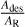 ], with Ades the pre-exponential factor of the adsorbed molecule (s−1), AR the pre-exponential factor of the adsorbed molecule ionisation (s−1), and ΔE the difference in the activation energies from the Arrhenius equations controlling the adsorption of molecules onto the solid and the ionisation of the adsorbed molecule onto the solid surface (J.mol−1). R is the ideal gas constant and T(z) the atmospheric temperature at altitude z (Berland et al. 1997; Taysum et al. 2024).
], with Ades the pre-exponential factor of the adsorbed molecule (s−1), AR the pre-exponential factor of the adsorbed molecule ionisation (s−1), and ΔE the difference in the activation energies from the Arrhenius equations controlling the adsorption of molecules onto the solid and the ionisation of the adsorbed molecule onto the solid surface (J.mol−1). R is the ideal gas constant and T(z) the atmospheric temperature at altitude z (Berland et al. 1997; Taysum et al. 2024).
θLang is the water ice surface coverage as defined by Kippenberger et al. (2019): ![$\theta_{\text{Lang}} = \frac{K_{\text{Lang}}[\text{HCl}]}{1+K_{\text{Lang}}[\text{HCl}]}$](/articles/aa/full_html/2025/07/aa53872-25/aa53872-25-eq72.png) , with KLang = 9.6 × 10−11 cm3.mol−1 and [HCl] the number density of HCl (mol.cm−3).
, with KLang = 9.6 × 10−11 cm3.mol−1 and [HCl] the number density of HCl (mol.cm−3).
[20] - Zhang et al. (2022), [21] - Seisel et al. (2005). For other references, see footnote of Table A.2
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


