Table A.2
Chlorine photochemical scheme included in the MPCM
| Label | Reaction | Reaction rate | Reference |
|---|---|---|---|
| ph1 | HCl + hν → H + Cl | [1] | |
| ph2 | Cl2 + hν → Cl + Cl | [2] | |
| ph3 | ClO + hν → Cl + O(1D) | [2] | |
| ph4 | ClO + hν → Cl + O | [2] | |
| ph5 | HOCl + hν → Cl + OH | [2] | |
| ph6 | ClOO + hν → Cl + O2 | [2] | |
| ph7 | OClO + hν → ClO + O | [2] | |
| h1 | 6 H2O + dust → Cl + 6 H2O | 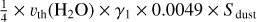 |
[3] based on [4] |
| h2 | 2 HCl + dust → 2 H | 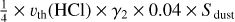 |
[3], based on [6] and [7] |
| h3 | HCl + water ice → H + Cl or H | 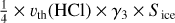 |
[3] based on [8] and [9] |
| h4 | Cl2 + dust → Ø | 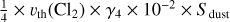 |
[3] based on [2] |
| h5 | ClO + water ice → O | 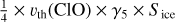 |
[3], based on [2] \\ \hline |
| cl1 | Cl + O3 → ClO + O2 | 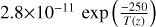 |
[10] |
| cl2 | ClO + O → Cl + O2 | 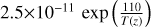 |
[10] |
| cl3 | ClO + ClO → Cl2 + O2 | 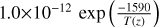 |
[10] |
| cl4 | ClO + ClO → 2 Cl + O2 | 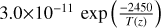 |
[10] |
| cl5 | ClO + ClO → Cl + OClO | 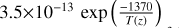 |
[10] |
| cl6 | ClO + ClO + M → Cl2O2 + M | ![\begin{tabular}[c]{@{}l@{}}$k_0 = 1.9\times 10^{-32} \, \left ( \frac{298}{T(z)} \right )^{3.6}$\\ $k_{\infty} = 3.7\times 10^{-12} \, \left ( \frac{298}{T(z)} \right )^{1.6}$\end{tabular}](/articles/aa/full_html/2025/07/aa53872-25/aa53872-25-eq24.png) |
[10] |
| cl7 | Cl2O2 → ClO + ClO | 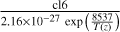 |
[10] |
| cl8 | Cl + H2 → HCl + H | 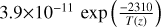 |
[10] |
| cl9 | Cl + HO2 → HCl + O2 | 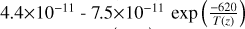 |
[10] |
| cl10 | Cl + HO2 → ClO + OH | 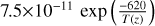 |
[10] |
| cl11 | Cl + H2O2 → HCl + HO2 | 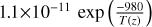 |
[10] |
| cl12 | ClO + OH → Cl + HO2 | 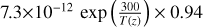 |
[10] |
| cl13 | ClO + OH → HCl + O2 | 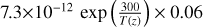 |
[10] |
| cl14 | ClO + HO2 → HOCl + O2 | 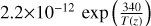 |
[10] |
| cl15 | HCl + OH → Cl + H2O | 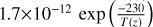 |
[10] |
| cl16 | HOCl + OH → ClO + H2O | 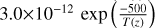 |
[10] |
| cl17 | CO + Cl + M → ClCO + M | ![2.5\, {[}M{]} \, 1.3$\times 10^{-33} \, \left ( \frac{300}{T(z)} \right )^{3.8}$](/articles/aa/full_html/2025/07/aa53872-25/aa53872-25-eq35.png) |
[11] |
| cl18 | ClOO + Cl → ClO + ClO | 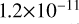 |
[2] |
| cl19 | ClOO + Cl → O2 + Cl2 | 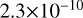 |
[2] |
| cl20 | ClOO + M → Cl + O2 + M | ![2.8$\times 10^{-10} \, \exp{\left ( \frac{-1820}{T(z)} \right )}$ \, {[}M{]}](/articles/aa/full_html/2025/07/aa53872-25/aa53872-25-eq38.png) |
[11] |
| cl21 | HClO4 + OH → ClO4 + H2O | 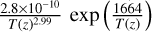 |
[12] |
| cl22 | Cl + O2 + M → ClOO + M | ![2.5\, {[}M{]} \, 1.4$\times 10^{-33} \, \left ( \frac{300}{T(z)} \right )^{3.9}$](/articles/aa/full_html/2025/07/aa53872-25/aa53872-25-eq40.png) |
[11] |
| cl23 | Cl + Cl2O2 → Cl2 + ClOO | 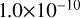 |
[11] |
| cl24 | ClCO → CO + Cl | ![{[}M{]} \, 4.1$\times 10^{-10} \, \exp{\left ( \frac{-2960}{T(z)} \right )}$](/articles/aa/full_html/2025/07/aa53872-25/aa53872-25-eq42.png) |
[11] |
| cl25 | Cl2 + O($^1$D) → Cl2 + O | ![{[}Cl$_2${]} \, $\frac{1}{4} \times 2.7\times 10^{-10}$](/articles/aa/full_html/2025/07/aa53872-25/aa53872-25-eq43.png) |
[2] |
| cl26 | Cl2 + O($^1$D) → Cl + ClO | 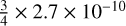 |
[2] |
| cl27 | Cl2 + OH → HOCl + Cl | 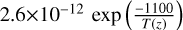 |
[2] |
| cl28 | Cl2 + H → HCl + Cl | 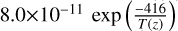 |
[13] |
| cl29 | HCl + O(1D) → HCl + O | ![0.12 $\times 1.5\times 10^{-10}$ \, {[}HCl{]}](/articles/aa/full_html/2025/07/aa53872-25/aa53872-25-eq47.png) |
[2] |
| cl30 | HCl + O(1D) → ClO + H | 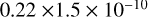 |
[2] |
| cl31 | HCl + O(1D) → Cl + OH | 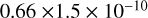 |
[2] |
| cl32 | HCl + O → OH + Cl | 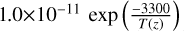 |
[2] |
| cl33 | HCl + H → H2 + Cl | 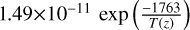 |
[14] |
| cl34 | HOCl + O → ClO + OH | 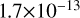 |
[11] |
| cl35 | Cl + O3 + M → ClO3 + M | ![2.5 \, {[}M{]} \, $\times 1.0\times 10^{-31}$](/articles/aa/full_html/2025/07/aa53872-25/aa53872-25-eq53.png) |
[15], based on [16] |
| cl36 | ClO + ClO3 → ClOO + OClO | 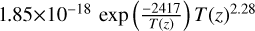 |
[15], based on [17] |
| cl37 | ClO + ClO3 → OClO + OClO | 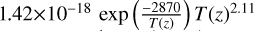 |
[15], based on [17] |
| cl38 | ClO + ClO3 + M → Cl2O4 + M | ![\begin{tabular}[c]{@{}l@{}}$k_0 = \frac{1.43\times 10^{-1}}{T(z)^{10.19}} \, \exp{\left ( \frac{-1597}{T(z)} \right )}$\\ $k_{\infty} = 1.43\times 10^{-10} \, \exp{\left ( \frac{-82}{T(z)} \right )} \, T(z)^{0.094}$\end{tabular}](/articles/aa/full_html/2025/07/aa53872-25/aa53872-25-eq56.png) |
[17] |
| cl39 | ClO3 + OH → HClO4 | 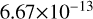 |
[15], based on [16] |
| cl40 | ClO3 + OH + M → HClO4 + M | ![\begin{tabular}[c]{@{}l@{}}$k_0 = \frac{1.94\times 10^{36}}{T(z)^{15.3}} \, \exp{\left ( \frac{-5542}{T(z)} \right )}$\\ $k_{\infty} = 3.2\times 10^{-10} \, \exp{\left ( \frac{-25}{T(z)} \right )} \, T(z)^{0.07}$\end{tabular}](/articles/aa/full_html/2025/07/aa53872-25/aa53872-25-eq58.png) |
[15], based on [18] |
| cl41 | ClO3 + OH → OClO + HO2 | 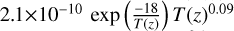 |
[15], based on [18] |
| cl42 | OClO + O + M → ClO3 + M | ![\begin{tabular}[c]{@{}l@{}}$k_0 = 3.0\times 10^{-31} \, \left ( \frac{298}{T(z)} \right )^{3.1}$\\ $k_{\infty} = 8.3\times 10^{-12} \, \left ( \frac{298}{T(z)} \right )$\end{tabular}](/articles/aa/full_html/2025/07/aa53872-25/aa53872-25-eq60.png) |
[2] |
| cl43 | OClO + O3 → ClO3 + O2 | 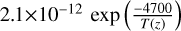 |
[2] |
| cl44 | ClO4 + Cl → ClO3 + ClO | 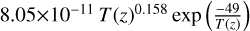 |
[17] |
| cl45 | ClO4 + HOCl → ClO + HClO4 | 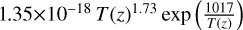 |
[19] |
| cl46 | HOCl+ Cl → Cl2 + OH | 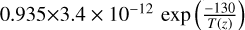 |
[2] and [15] |
| cl47 | HOCl+ Cl → HCl + ClO | 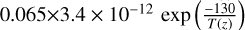 |
[2] and [15] |
| cl48 | OClO+ O → ClO + O2 | 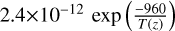 |
[11] |
| cl49 | OClO+ OH → HOCl + O2 | 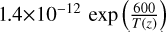 |
[2] |
| cl50 | OClO+ Cl → ClO + ClO | 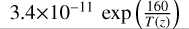 |
[2] |
Notes. Values between brackets are number densities in cm−3. [M] is the number density of the third body for three-body reactions. Sdust and Sice are the surface area of dust and water ice, respectively, in cm−1.
vth(species) is the thermal speed of the considered species in cm/s, computed as 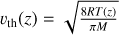 , with R the ideal gas constant, T(z) the temperature at altitude level z and M the molar mass of the considered species in g/mol.
, with R the ideal gas constant, T(z) the temperature at altitude level z and M the molar mass of the considered species in g/mol.
[1] - Heays et al. (2017), [2] - Burkholder et al. (2019), [3] - Taysum et al. (2024), [4] - Keller et al. (2006), [5] - Boynton et al. (2009), [6] - Huynh & McNeill (2020), [7] - Huynh & McNeill (2021), [8] - Hynes et al. (2001), [9] - Kippenberger et al. (2019), [10] - Sander et al. (2019), [11] -Atkinson et al. (2007), [12] - Zhu & Lin (2010), [13] - Berho et al. (1999), [14] - Neufeld & Wolfire (2009), from the KIDA database (Wakelam et al. 2012), [15] - Catling et al. (2010), [16] - Simonaitis & Heicklen (1975), [17] - Xu & Lin (2003), [18] - Zhu & Lin (2001), [19] - Xu & Lin (2010)
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


