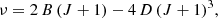| Issue |
A&A
Volume 688, August 2024
|
|
|---|---|---|
| Article Number | L29 | |
| Number of page(s) | 5 | |
| Section | Letters to the Editor | |
| DOI | https://doi.org/10.1051/0004-6361/202451319 | |
| Published online | 15 August 2024 | |
Letter to the Editor
Space and laboratory discovery of iminopentadienylidene, HNC5⋆
1
Dept. de Astrofísica Molecular, Instituto de Física Fundamental (IFF-CSIC), C/ Serrano 121, 28006 Madrid, Spain
2
Department of Applied Chemistry, Science Building II, National Yang Ming Chiao Tung University, 1001 Ta-Hsueh Rd., Hsinchu 300098, Taiwan
3
Observatorio de Yebes (IGN), Cerro de la Palera s/n, 19141 Yebes, Guadalajara, Spain
4
Observatorio Astronómico Nacional (OAN, IGN), C/ Alfonso XII, 3, 28014 Madrid, Spain
Received:
1
July
2024
Accepted:
19
July
2024
We report the discovery of HNC5 in TMC-1. Six lines have been found in harmonic relation, with quantum numbers J = 12−11 up to J = 17−16. The lines can be reproduced with the standard frequency relation for linear molecules with B = 1361.75034 ± 0.00033 MHz and D = 32.2 ± 0.7 Hz. The assignment of the carrier to iminopentadienylidene was achieved through examining the possible candidates at a high level of theoretical ab initio calculations. Motivated by the good agreement between the observed B and the calculated value for HNC5, we searched for it in the laboratory and observed the transitions J = 5−4 to 7−6. The derived rotational and distortion constants are 1361.74998 ± 0.00040 MHz and 26.5 ± 5.5 Hz, respectively. Hence, we solidly conclude that the carrier of the lines found in TMC-1 is HNC5. The calculated dipole moment for this species is 7.7 D and the derived column density is (1.3 ± 0.2) × 1010 cm−2. We used the new QUIJOTE data to improve previous observations of HC4NC and found that the abundance ratio HC4NC/HNC5 is 10 ± 2. The abundance ratio of HC5N and its two isomers HC4NC and HNC5 is 500 ± 80 and 5100 ± 800, respectively. These abundance ratios are higher by a factor of ∼10 than those of the equivalent isomers of HC3N. Chemical models reproduce the observed abundances reasonably well when a chemistry similar to that of the smaller species C3HN isomers is adopted. The formation of HNC5 and HC4NC arises from the dissociative recombination with electrons of the cations HC5NH+ and HC4NCH+.
Key words: astrochemistry / line: identification / molecular data / ISM: molecules / ISM: individual objects: TMC-1
© The Authors 2024
 Open Access article, published by EDP Sciences, under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Open Access article, published by EDP Sciences, under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article is published in open access under the Subscribe to Open model. Subscribe to A&A to support open access publication.
1. Introduction
The TMC-1 dark cloud has proven to be a vital source for studying the chemistry of the interstellar medium. About 70 new molecules have been detected in recent years. These discoveries allow us to refine the chemical models of cold clouds, including molecules that were not originally expected.
One of the most important of all the families of molecules detected in TMC-1 is the series of cyanopolyynes HC2n + 1N. The largest series detected to date is HC11N (Bell et al. 1997; Loomis et al. 2016; Cordiner et al. 2017; Loomis et al. 2021). The high abundance of these species has allowed the detection of their higher-energy neutral isomers HNCCC (Kawaguchi et al. 1992a), HCCNC (Kawaguchi et al. 1992b), and HC4NC (Cernicharo et al. 2020; Xue et al. 2020). Their protonated forms HCCCNH+ (Kawaguchi et al. 1994), HCCNCH+ (Agúndez et al. 2022), HC5NH+ (Marcelino et al. 2020), and HC7NH+ (Cabezas et al. 2022) have also been reported. Several of these species have been detected with the Q-band Ultrasensitive Inspection Journey to the Obscure TMC-1 Environment (Cernicharo et al. 2021, QUIJOTE). This survey has permitted us to detect the isotopologues of HCCNC and HNCCC (Cernicharo et al. 2024a) and the doubly substituted isotopologues of HC3N (Tercero et al. 2024). Moreover, its sensitivity has recently also permitted us to discover several radical cations related to cyanopolyynes, such as HC3N+, HC5N+, and HC7N+. They have been fully spectroscopically characterized in TMC-1 (Cabezas et al. 2024; Cernicharo et al. 2024b).
When all these discoveries are taken into account, we expect to detect other isomers of the cyanopolyyne family, in particular, those of HC5N and HC7N. In this Letter, we report the detection of a series of lines in TMC-1 that we assign to HNC5. This assignment is based on a high level of theory ab initio calculations and laboratory observation of three of its rotational transitions. We compare its abundance to that of HC5N and analyse their abundance ratio in the context of the most advanced chemical modelling.
2. Observations
The observational data presented in this work are part of the QUIJOTE spectral line survey (Cernicharo et al. 2021) in the Q band towards TMC-1(CP) (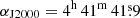 and δJ2000 = +25° 41′ 27.0″), which was performed at the Yebes 40m radio telescope. This survey was made using a receiver that was built within the Nanocosmos project1, which consists of two cooled high electron mobility transistor (HEMT) amplifiers covering the 31.0−50.3 GHz band with horizontal and vertical polarizations. Fast Fourier transform spectrometers (FFTSs) with 8 × 2.5 GHz with a spectral resolution of 38.15 kHz provide the whole coverage of the Q band in both polarizations. The receiver temperatures are 16 K at 32 GHz and 30 K at 50 GHz. The experimental setup was described in detail by Tercero et al. (2021).
and δJ2000 = +25° 41′ 27.0″), which was performed at the Yebes 40m radio telescope. This survey was made using a receiver that was built within the Nanocosmos project1, which consists of two cooled high electron mobility transistor (HEMT) amplifiers covering the 31.0−50.3 GHz band with horizontal and vertical polarizations. Fast Fourier transform spectrometers (FFTSs) with 8 × 2.5 GHz with a spectral resolution of 38.15 kHz provide the whole coverage of the Q band in both polarizations. The receiver temperatures are 16 K at 32 GHz and 30 K at 50 GHz. The experimental setup was described in detail by Tercero et al. (2021).
All observations are performed using frequency-switching observing mode with a frequency throw of 10 and 8 MHz. The total observing time on the source for data taken with frequency throws of 10 MHz and 8 MHz is 465 and 737 hours, respectively. Hence, the total observing time on source is 1202 hours. The QUIJOTE sensitivity varies between 0.08 mK at 32 GHz and 0.2 mK at 49.5 GHz, and this is about 50 times better than that of previous line surveys in the Q band of TMC-1 (Kaifu et al. 2004). A detailed description of the line survey and the data analysis procedure we followed was provided in Cernicharo et al. (2021, 2022). The main-beam efficiency can be given across the Q band by Beff = 0.797 exp[−(ν(GHz)/71.1)2]. The forward telescope efficiency is 0.97. The telescope beam size at half-power intensity is 54.4″ at 32.4 GHz and 36.4″ at 48.4 GHz. The absolute calibration uncertainty is 10%. The data were analysed with the GILDAS package2.
3. Results
3.1. Series of lines B1362
In the unknown features of the line survey, we found six lines in harmonic relation with integer quantum numbers. The upper rotational quantum numbers (Ju) range from 12 to 17. The lines are shown in the left panels of Fig. 1. Their line parameters are given in Table 1. The frequencies of the observed lines can be fitted with the standard relation
 |
Fig. 1. Observed transitions of the isomers of HC5N in TMC-1. The lines of HNC5 are shown in the left panels, and the lines of HC4NC are shown in the right panels. The abscissa corresponds to the rest frequency, and the ordinate is the antenna temperature, corrected for atmospheric and telescope losses, in milli Kelvin. The blank channels correspond to negative features produced when folding the frequency-switched data. The red lines correspond to the synthetic spectra calculated for a column density of 1.3 × 1010 cm−2 for HNC5 (Trot = 6 K), and of 1.3 × 1011 cm−2 for HC4NC (Trot = 7.5 K). |
Observed line parameters of HNC5 and HC4NC.
with B = 1361.75034 ± 0.00033 MHz and D = 32.2 ± 0.7 Hz. The root mean square (rms) of the fit is 5.1 kHz. We refer to this series of lines as B1362. Fig. 1 shows that the line intensity declines by a factor of 4 between Ju = 12 and Ju = 17, which indicates a moderate rotational temperature and probably a high dipole moment for the molecule. Except for the J = 17−16 transition of B1362, which is marginally detected, all the other lines are detected with a high signal-to-noise ratio (S/N). The derived rotational constant is between that of HC5N (B ∼ 1331.3 MHz; Bizzocchi et al. 2004) and that of HC4NC (B ∼ 1401.2 MHz; Botschwina et al. 1998). The distortion constant of B1362 is almost identical to that of these two species, 30.1 and 32.7 Hz, respectively. The carrier could be an isotopologue of HC4NC. However, the observed intensities do not match this possibility. The lines of HC4NC are shown in the right panels of Fig. 1 and show that the line intensity ratio of this species and B1362 is ∼2. The abundances of none of the isotopologues of HC4NC are expected to produce intensities as high as this. Moreover, ab initio calculations permitted us to estimate their rotational constants at an accuracy of 0.05%, and none of them match the rotational constant B of B1362.
A molecule that could fit our rotational constant is HNC5. As this species is quasi-linear, the constant B derived from the observed transitions in Eq. (1) corresponds to (B + C)/2 for Ka = 0. Its rotational constant was calculated at the MP2/cc-pVTZ level of theory to be ∼1348.3 MHz (see Table C.1 of Cernicharo et al. 2020). Applying a correction factor of 1.009 derived from the calculated and observed rotational constant of HC5N in the same work, we estimate a value of 1360 MHz for the rotational constant of HNC5. More accurate values were estimated, as shown in Sect. 3.2, that approach the rotational constant of B1362 very closely. Motivated by this good agreement, we searched for and detected this species in the laboratory (see Sect. 3.2).
The line parameters for HC4NC are given in Table 1 (see Fig. 1). The new QUIJOTE dataset provides a more sensitive spectrum than was used in Cernicharo et al. (2020), allowing the detection of a new transition (J = 17 − 16; see Fig. 1). The new frequency determinations in TMC-1 for HC4NC were merged with those from the laboratory (Botschwina et al. 1998) and were fitted with the standard Hamiltonian of a linear molecule with a hyperfine structure (from the laboratory data for low-J lines). We derived a new set of molecular parameters for this isomer, B = 1401.182158 ± 35 MHz, D = 32.73 ± 20 Hz, and eQq = 0.970 ± 19 MHz, which is very similar to but more acurrate than those obtained by Cernicharo et al. (2020). The rms of the fit is 3.2 kHz.
3.2. Quantum chemical calculations and laboratory detection of HNC5
The geometry optimization calculations were carried out at the CCSD(T)-F12/cc-pCVTZ-F12 level of theory (Raghavachari et al. 1989; Adler et al. 2007; Knizia et al. 2009; Hill et al. 2010; Hill & Peterson 2010) using the program Molpro 2024.1 (Werner et al. 2024). Our quantum chemical calculations show that iminopentadienylidene is a quasi-linear molecule (see Fig. 2). The values for the calculated rotational constants Ae, Be, and Ce are 1167.6, 1.36095, and 1.35937 GHz. To obtain accurate spectroscopic parameters, we performed calculations for the well-known isomer HC5N, for which the rotational parameters were determined experimentally. Using the same calculation level, we obtained a value for the constant Be for HC5N of 1329.6 MHz, and the experimental value is 1331.33269 MHz. Applying the ratio of experimental and theoretical results, the predicted value for the Beff of HNC5 is 1361.9 MHz, which agrees excellently with that of B1362. Furthermore, this procedure allowed us to recover the calculation error on Beff and the zero-point vibrational contribution. The dipole moment at the CCSD(T)-F12/cc-pCVTZ-F12 optimized geometry for HNC5 is 7.7D. Additionally, the nuclear electric quadrupole constant eQq for the nitrogen nucleus was also calculated using the quadratic configuration interaction with the single and double excitation (QCISD; Pople et al. 1987) level of calculation with the polarized valence triple-ζ basis set (cc-pVTZ; Woon & Dunning 1993). The calculated value is 0.99 MHz. This calculation was made using the Gaussian16 program (Frisch 2016).
 |
Fig. 2. Optimized molecular structure of HNC5 as obtained from quantum chemical calculations. |
The rotational spectrum of HNC5 was measured using a Balle-Flygare-type Fourier transform microwave (FTMW) spectrometer combined with a pulsed discharge nozzle (Endo et al. 1994; Cabezas et al. 2016). The reactive transient species, HNC5, was produced in a supersonic expansion by a pulsed electric discharge of a gas mixture of CH2CHCN (0.2%) and C2H2 (0.5%) diluted in Ar and applying a voltage of 1500 V through the throat of the nozzle source. The rotational constants derived from the astronomical observations were used to predict the frequencies of the rotational transitions J′−J″ = 5 − 4, 6 − 5, and 7 − 6 of HNC5. Although three hyperfine components were expected for each rotational transition, only a single line was observed at the predicted frequencies (see Fig. 3). They were assigned to the strongest hyperfine component of each transition, and their frequencies are given in Table 1. The observed frequencies were fit using the SPFIT program (Pickett 1991), and the B and D molecular constants agree well with those obtained from the fit using the observed lines from TMC-1. A merged fit to the laboratory and TMC-1 data provides improved values for these parameters. The eQq constant was fixed in the fit to the calculated value. In this fit, we considered uncertainties of 2 kHz for the laboratory lines and uncertainties of 10 kHz for the transitions observed in TMC-1. The derived molecular constants from the different fits are given in Table 2. The almost identical sets of molecular constants obtained from TMC-1 lines and from the laboratory, as well as the good agreement with the values obtained from quantum chemical calculations, allow us to conclude that the carrier of our series of lines is HNC5.
 |
Fig. 3. Fourier transform microwave spectra of HNC5 showing the J = 5 − 4 rotational transition. The spectrum was achieved through 10 000 accumulation shots at a repetition rate of 10 Hz. The coaxial arrangement of the adiabatic expansion and the resonator axis produces an instrumental Doppler doubling. The resonance frequency is calculated as the average of the two Doppler components. |
Molecular constants of HNC5.
4. Discussion
4.1. Column densities
Using the dipole moment of 7.7 D calculated in Sect. 3.2, we fitted the observed intensities and line profiles of HNC5. We adopted a source with a uniform brightness temperature and a diameter of 80″ (Fossé et al. 2001) and derived a rotational temperature of 6.0 ± 0.5 K and a column density of (1.3 ± 0.2) × 1010 cm−2. HC4NC was previously detected with the QUIJOTE data of 2020 by Cernicharo et al. (2020). We revised the observed intensities of this isomer using the same version of the line survey as for HNC5. These data are five times more sensitive than those used by Cernicharo et al. (2020). The lines of HC4NC are shown in the right panels of Fig. 1, and their line parameters are given in Table 1. The adopted dipole moment for this isomer is 3.25 D (Botschwina et al. 1998). We derive a rotational temperature of 7.5 ± 0.3 K and a column density of (1.3 ± 0.2) × 1011 cm−2. The H2 column density for TMC-1 is 1022 cm−2 (Cernicharo & Guélin 1987).
The column density of HC4NC is lower by almost a factor of two than that of Cernicharo et al. (2020). This factor comes from the limited S/N that was achieved with the first QUIJOTE data, and in particular, from the intensities observed for the J = 15−14 and 16−15 transitions at that time, which are too high and produced a high rotational temperature of 9.8 ± 0.9 K and a higher column density to fit these lines.
The column density of HC5N has recently been revisited (Cernicharo et al., in prep.) and was determined to be (6.6 ± 0.1) × 1013 cm−2. Hence, the HC5N/HNC5 abundance ratio is 5100 ± 800, and the abundance ratio of HC4NC and HNC5 is 10 ± 2. These abundance ratios are given in Table 3, together with those of the HC3N isomers (Cernicharo et al. 2024a; Tercero et al. 2024). If the observed tendency for the decrease in the abundance of the isomers with the size of the molecule applies to the next members of the cyanopolyyne family of molecules, then HC6NC and HNC7 are well below the current detection limit of QUIJOTE.
Column densities of the isomers of HC3N and HC5N.
4.2. Chemical model
Current chemical models of cold dense clouds (Agúndez & Wakelam 2013) attribute the formation of HCCNC and HNC3, the metastable isomers of HC3N, to the dissociative recombination of the cation HC3NH+. Theoretical calculations by Osamura et al. (1999) indicated that a rearrangement of the skeleton of heavy atoms is possible, so that in addition to the production of HC3N + H, the channels HCCNC + H and HNC3 + H are also energetically allowed. The dissociative recombination of the deuterated variant of HC3NH+ was measured by Geppert et al. (2004), and some information is available on the product channels. Specifically, the channels that lead to C3HN + H and C2N + CN + H account for 52% of the reaction products. However, there is no information on the relative contributions of each C3HN isomer, but this would be of great interest because it is thought to be the main factor that regulates the relative abundances of the different C3HN isomers in cold clouds.
Unlike in the case of HCN and HNC, which are observed to have similar abundances in cold clouds because they are formed with similar yields in the dissociative recombination of HCNH+ (HCN/HNC = 3, according to the measurements by Amano et al. 2008), the metastable isomers HCCNC and HNC3 are observed to be much less abundant than HC3N in cold clouds (see Table 3), which implies that they are probably formed with lower branching ratios than HC3N in the dissociative recombination of HC3NH+. Interestingly, the theoretical study by Osamura et al. (1999) indicated that a fourth isomer, HCNCC (not yet identified in space), might also be formed during the dissociative recombination of HC3NH+. Alternatively, the different C3HN isomers might also form with similar yields in the dissociative recombination of HC3NH+, and the lower abundance of the metastable isomers with respect to the most stable one HC3N might be due to a enhanced reactivity. To complicate this picture slightly, isomers of HC3NH+, such as HCCNCH+, could also act as precursors of C3HN isomers, although their contribution in current chemical models is lower than the HC3NH+ + e− pathway. In summary, the scenario that is currently proposed to explain the presence and relative abundances of the different isomers of HC3N still requires validation from a study of the different chemical processes at work, namely, the relative yields forming the different C3HN isomers in the dissociative recombination of HC3NH+ and the reactivity of the different C3HN isomers with abundant reactive species, such as neutral atoms and cations.
The chemistry of C5HN isomers is likely to be similar to that of C3HN. The latest release of the UMIST database for astrochemistry (Millar et al. 2024) includes HC5N and the metastable isomer HC4NC, following its discovery in space (Cernicharo et al. 2020). The main routes to these species in the UMIST network involve the dissociative recombination of HC5NH+, and in the case of HC4NC, also the dissociative recombination of HC4NCH+. We included HNC5 as a new species, with formation and destruction routes similar to those included for HC4NC. That is, HNC5 would be formed by the dissociative recombination of HC5NH+ and HC4NCH+, while it would be mainly destroyed through reactions with abundant cations, such as C+, HCO+, 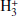 , He+, and N2H+. Under the assumption of a similar reactivity for all C5HN isomers, the relevant parameters that regulate the relative abundances are the branching ratios that yield each isomer in the dissociative recombination of HC5NH+, and to a lower extent, those of HC4NCH+. We assumed HC5N:HC4NC:HNC5 yields of 100:0.1:0.1 in the dissociative recombination of HC5NH+ and 1:100:1 for HC4NCH+. In the case of the C3HN isomers, the HC3N:HCCNC:HNC3 yields adopted in the UMIST network are 100:10:10 for HC3NH+ and 20:100:20 for HCCNCH+. These yields are not based on theoretical or experimental studies, but are rather estimates inspired by the relative abundances observed in space. It is clear from the relative abundances given in Table 3 that the relative abundances of the metastable isomers with respect to the most stable isomer decrease considerably (by about a factor of 10) as the size increases. This could be an indication of a lower yield of metastable isomers in the dissociative recombination of HC5NH+ compared to that of HC3NH+. It would be interesting to investigate this.
, He+, and N2H+. Under the assumption of a similar reactivity for all C5HN isomers, the relevant parameters that regulate the relative abundances are the branching ratios that yield each isomer in the dissociative recombination of HC5NH+, and to a lower extent, those of HC4NCH+. We assumed HC5N:HC4NC:HNC5 yields of 100:0.1:0.1 in the dissociative recombination of HC5NH+ and 1:100:1 for HC4NCH+. In the case of the C3HN isomers, the HC3N:HCCNC:HNC3 yields adopted in the UMIST network are 100:10:10 for HC3NH+ and 20:100:20 for HCCNCH+. These yields are not based on theoretical or experimental studies, but are rather estimates inspired by the relative abundances observed in space. It is clear from the relative abundances given in Table 3 that the relative abundances of the metastable isomers with respect to the most stable isomer decrease considerably (by about a factor of 10) as the size increases. This could be an indication of a lower yield of metastable isomers in the dissociative recombination of HC5NH+ compared to that of HC3NH+. It would be interesting to investigate this.
The calculated abundances of C3HN and C5HN isomers are shown as a function of time in Fig. 4. The peak calculated abundances that were reached at a time of a few times 105 yr clearly agree within one order of magnitude with the values observed in TMC-1. This fact supports the scenario that metastable isomers of cyanopolyynes form through the dissociative recombination of the protonated cyanopolyyne precursor. We caution, however, that the rate coefficients we used, and especially the branching ratios we adopted, are very speculative and require a more solid theoretical or experimental basis.
 |
Fig. 4. Calculated fractional abundances for the C3HN (top panel) and C5HN isomers (bottom panel) as a function of time. The horizontal dotted lines correspond to the values observed in TMC-1 adopting the column densities of Table 3 and a column density of H2 of 1022 cm−2 (Cernicharo & Guélin 1987). |
5. Conclusions
We reported the discovery of HNC5 in TMC-1 based on the detection of six rotational transitions that range from 1.6 to 0.4 mK, a column density of (1.3 ± 0.2) × 1010 cm−2, and a rotational temperature of 6.0 ± 0.5 K. This is the second isomer of cyanodiacetylene that was detected in this source. We performed both a theoretical and laboratory study to report the first data of the rotational constants of this species. The abundance ratio of HC4NC and HNC5 is 10 ± 2, and the ratio of HC5N and HNC5 is 5100 ± 800. A comparison of these abundance ratios with those of the isomers of HC3N indicates that the ratios decrease by a factor of 10 between these two consecutive cyanopolyynes. We modelled the chemistry of the different isomers of HC3N and HC5N and conclude that the calculated abundances are similar to the observed ones within one order magnitude.
Acknowledgments
The present study was supported by Ministry of Science and Technology of Taiwan and Consejo Superior de Investigaciones Científicas for funding support under the MoST-CSIC Mobility Action 2021 (Grant 11-2927-I-A49-502 and OSTW200006). R.F., C.C., M.A, and J.C. thank Ministerio de Ciencia e Innovación of Spain (MICIU) for funding support through projects PID2019-106110GB-I00, PID2019-107115GB-C21/AEI/10.13039/501100011033, and PID2019-106235GB-I00. R.F., C.C., M.A, and J.C. also thank ERC for funding through grant ERC-2013-Syg-610256- 312 NANOCOSMOS. Y.E. acknowledges Ministry of Science and Technology of 313 Taiwan for MOST 104-2113-M-009-202 project.
References
- Adler, T. B., Knizia, G., & Werner, H.-J. 2007, J. Chem. Phys., 127, 221106 [Google Scholar]
- Agúndez, M., & Wakelam, V. 2013, Chem. Rev., 113, 8710 [Google Scholar]
- Agúndez, M., Cabezas, C., Marcelino, N., et al. 2022, A&A, 659, L9 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Amano, T., Zelinger, Z., Hirao, T., et al. 2008, J. Mol. Spectrosc., 251, 252 [NASA ADS] [CrossRef] [Google Scholar]
- Bell, M. B., Feldman, P. A., Travers, M. J., et al. 1997, ApJ, 483, L61 [NASA ADS] [CrossRef] [Google Scholar]
- Bizzocchi, L., Degli Esposti, C., & Botschwina, P. 2004, J. Mol. Spectrosc., 225, 145 [NASA ADS] [CrossRef] [Google Scholar]
- Botschwina, P., Heyl, Ä., Chen, W., et al. 1998, J. Chem. Phys., 109, 3108 [NASA ADS] [CrossRef] [Google Scholar]
- Cabezas, C., Guillemin, J.-C., & Endo, Y. 2016, J. Chem. Phys., 145, 184304 [Google Scholar]
- Cabezas, C., Agúndez, M., Marcelino, N., et al. 2022, A&A, 659, L8 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cabezas, C., Agúndez, M., Marcelino, N., et al. 2024, A&A, 687, L22 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., & Guélin, M. 1987, A&A, 176, 299 [Google Scholar]
- Cernicharo, J., Marcelino, N., Agúndez, M., et al. 2020, A&A, 642, L8 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Agúndez, M., Kaiser, R. I., et al. 2021, A&A, 652, L9 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Fuentetaja, R., Agúndez, M., et al. 2022, A&A, 663, L9 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Cabezas, C., Agúndez, M., et al. 2024a, A&A, 686, L15 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Tercero, B., Cabezas, C., et al. 2024b, A&A, 682, L13 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cordiner, M. A., Charnley, S. B., Kisiel, Z., et al. 2017, ApJ, 850, 187 [NASA ADS] [CrossRef] [Google Scholar]
- Endo, Y., Kohguchi, H., & Ohshima, Y. 1994, Faraday Discuss., 97, 341 [Google Scholar]
- Fossé, D., Cernicharo, J., Gerin, M., & Cox, P. 2001, ApJ, 552, 168 [Google Scholar]
- Frisch, M. J. 2016, Gaussian 16 Revision A.03 [Google Scholar]
- Geppert, W. D., Ehlerding, A., Hellberg, F., et al. 2004, ApJ, 613, 1302 [NASA ADS] [CrossRef] [Google Scholar]
- Hill, J. G., & Peterson, K. A. 2010, Phys. Chem. Chem. Phys., 12, 10460 [Google Scholar]
- Hill, J. G., Mazumder, S., & Peterson, K. A. 2010, J. Chem. Phys., 132, 054108 [Google Scholar]
- Kaifu, N., Ohishi, M., Kawaguchi, K., et al. 2004, PASJ, 56, 69 [Google Scholar]
- Kawaguchi, K., Takano, S., Ohishi, M., et al. 1992a, ApJ, 396, L49 [CrossRef] [Google Scholar]
- Kawaguchi, K., Ohishi, M., Ishikawa, S.-I., et al. 1992b, ApJ, 386, L51 [NASA ADS] [CrossRef] [Google Scholar]
- Kawaguchi, K., Kasai, Y., Ishikawa, S.-I., et al. 1994, ApJ, 420, L95 [Google Scholar]
- Knizia, G., Adler, T. B., & Werner, H.-J. 2009, J. Chem. Phys., 130, 054104 [Google Scholar]
- Loomis, R. A., Shingledecker, C. N., Langston, G., et al. 2016, MNRAS, 463, 4175 [NASA ADS] [CrossRef] [Google Scholar]
- Loomis, R. A., Burkhardt, A. M., Shingledecker, C. N., et al. 2021, Nat. Astron., 5, 188 [NASA ADS] [CrossRef] [Google Scholar]
- Marcelino, N., Agúndez, M., Tercero, B., et al. 2020, A&A, 643, L6 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Millar, T. J., Walsh, C., Van de Sande, M., & Markwick, A. J. 2024, A&A, 682, A109 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Osamura, Y., Fukuzawa, K., Terzieva, R., & Herbst, E. 1999, ApJ, 519, 697 [NASA ADS] [CrossRef] [Google Scholar]
- Pickett, H. M. 1991, J. Mol. Spectrosc., 148, 371 [Google Scholar]
- Pople, J. A., Head-Gordon, M., & Raghavachari, K. 1987, J. Chem. Phys., 87, 5968 [NASA ADS] [CrossRef] [Google Scholar]
- Raghavachari, K., Trucks, G. W., Pople, J. A., & Head-Gordon, M. 1989, Chem. Phys. Lett., 157, 479 [Google Scholar]
- Tercero, F., López-Pérez, J. A., Gallego, J. D., et al. 2021, A&A, 645, A37 [EDP Sciences] [Google Scholar]
- Tercero, B., Marcelino, N., Roueff, E., et al. 2024, A&A, 682, L12 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Werner, H. J., Knowles, P. J., Knizia, G., et al. 2024, MOLPRO, Version 2024.1, A Package of ab initio Programs, https://www.molpro.net [Google Scholar]
- Woon, D. E., & Dunning, T. H. 1993, J. Chem. Phys., 98, 1358 [NASA ADS] [CrossRef] [Google Scholar]
- Xue, C., Willis, E. R., Loomis, R. A., et al. 2020, ApJ, 900, L10 [NASA ADS] [CrossRef] [Google Scholar]
All Tables
All Figures
 |
Fig. 1. Observed transitions of the isomers of HC5N in TMC-1. The lines of HNC5 are shown in the left panels, and the lines of HC4NC are shown in the right panels. The abscissa corresponds to the rest frequency, and the ordinate is the antenna temperature, corrected for atmospheric and telescope losses, in milli Kelvin. The blank channels correspond to negative features produced when folding the frequency-switched data. The red lines correspond to the synthetic spectra calculated for a column density of 1.3 × 1010 cm−2 for HNC5 (Trot = 6 K), and of 1.3 × 1011 cm−2 for HC4NC (Trot = 7.5 K). |
| In the text | |
 |
Fig. 2. Optimized molecular structure of HNC5 as obtained from quantum chemical calculations. |
| In the text | |
 |
Fig. 3. Fourier transform microwave spectra of HNC5 showing the J = 5 − 4 rotational transition. The spectrum was achieved through 10 000 accumulation shots at a repetition rate of 10 Hz. The coaxial arrangement of the adiabatic expansion and the resonator axis produces an instrumental Doppler doubling. The resonance frequency is calculated as the average of the two Doppler components. |
| In the text | |
 |
Fig. 4. Calculated fractional abundances for the C3HN (top panel) and C5HN isomers (bottom panel) as a function of time. The horizontal dotted lines correspond to the values observed in TMC-1 adopting the column densities of Table 3 and a column density of H2 of 1022 cm−2 (Cernicharo & Guélin 1987). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.