Fig. 10
Download original image
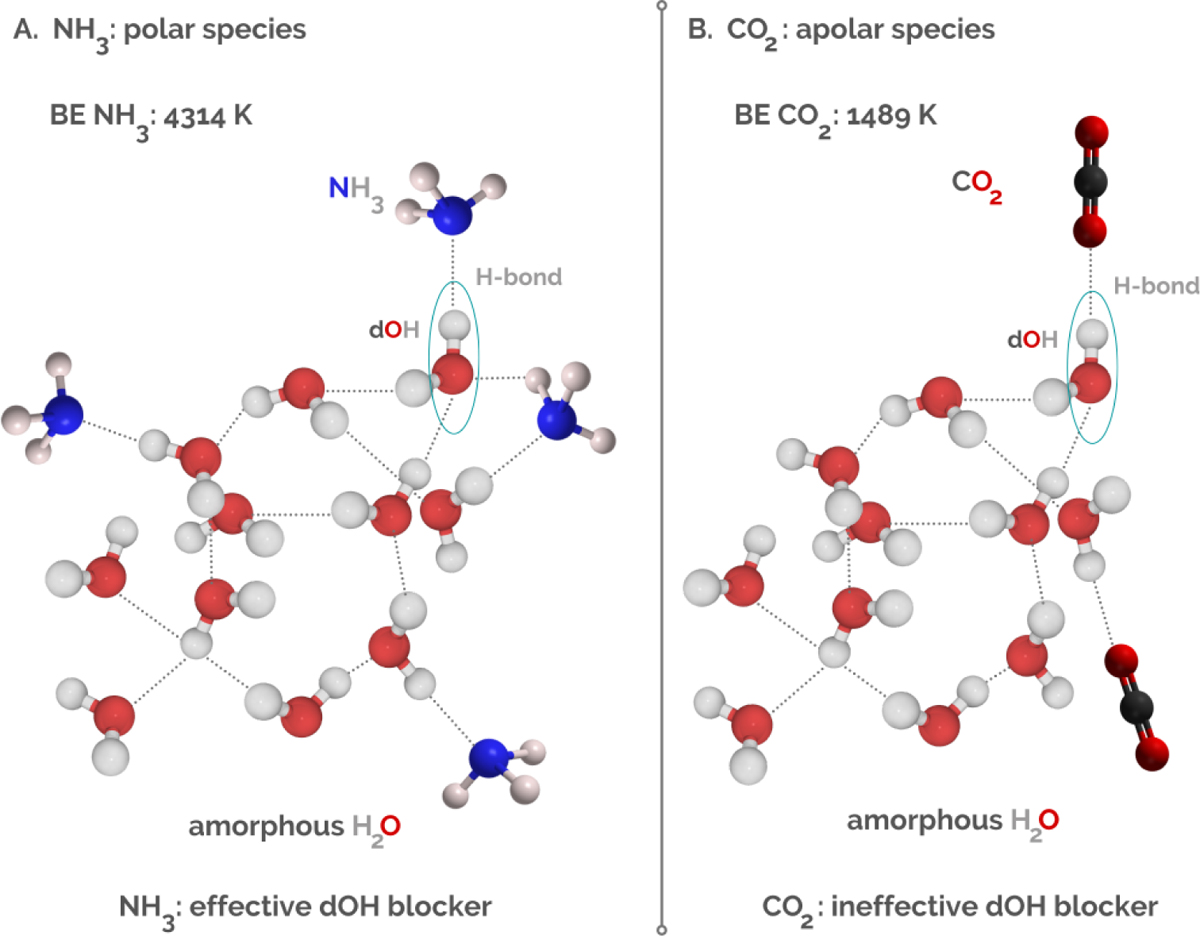
Schematic illustration of the interactions between (A) dOH and ammonia (NH3) and (B) dOH and carbon dioxide (CO2). the next three sentences appear to be interpretation or a comment and should be removed from the caption. Please check and remove if you agree NH3 is a polar molecule, it tightly binds to the dOH via H-bonding between the N atom and the dOH. CO2 is an apolar species, with binding energy three times lower than that of NH3. This implies that NH3 is a more effective dangling-OH band blocker over CO2. The reported binding energies are from Ferrero et al. (2020). The simplified geometries are an adaptation of real geometries obtained with quantum chemical calculations in Ferrero et al. (2020).
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


