Fig. 1.
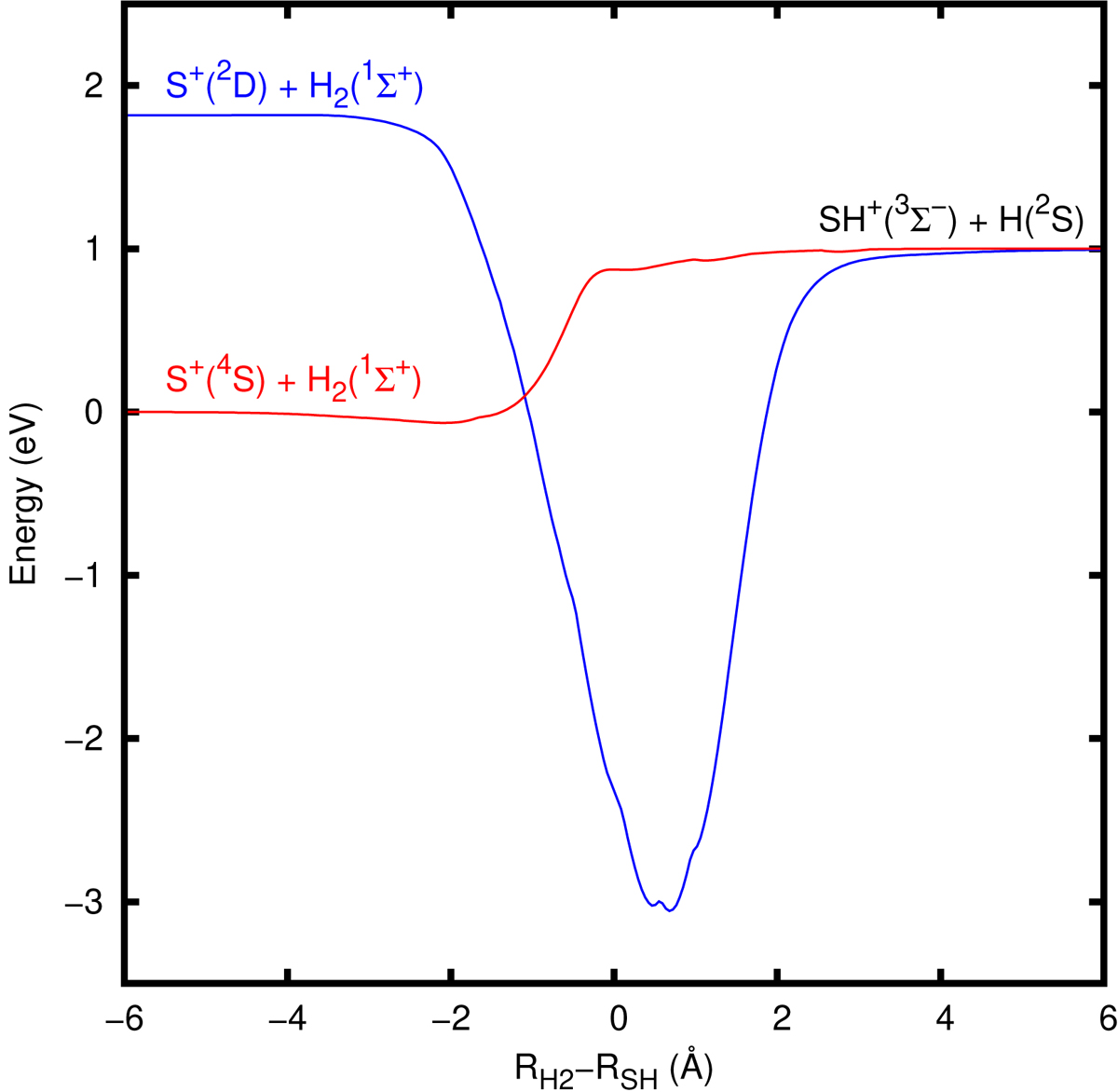
Minimum-energy path for the S++H2→ SH+(3Σ−) + H(2S) reaction considering the quartet and doublet states correlating to the SH+(3Σ−) asymptote. The abscissa is the reaction coordinate, defined as the difference of the H2 and SH distances, RH2 and RSH, respectively, in Å. The potential energies displayed correspond to the minimum of energy in the remaining two internal coordinates, i.e., the angle between the H–H and S–H bonds and the coordinate defined as (RH2 + RSH)/2. At distances of 6 Å, the interaction energy is lower than 1 meV, and cannot be appreciated in the figure.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


