| Issue |
A&A
Volume 603, July 2017
|
|
|---|---|---|
| Article Number | A139 | |
| Number of page(s) | 9 | |
| Section | Interstellar and circumstellar matter | |
| DOI | https://doi.org/10.1051/0004-6361/201731186 | |
| Published online | 25 July 2017 | |
Prebiotic molecules formation through the gas-phase reaction between HNO and CH2CHOH2+
1 Computational Chemistry Group, Departamento de Química Física y Química InorgánicaFacultad de Ciencias, Universidad de Valladolid, 47011 Valladolid, Spain
e-mail: predondo@qf.uva.es
2 Computational Chemistry Group, Departamento de Química Orgánica, Escuela de Ingenierías Industriales, Universidad de Valladolid, 47011 Valladolid, Spain
Received: 17 May 2017
Accepted: 15 June 2017
Context. Knowing how the molecules that are present in the ISM can evolve to more complex ones is an interesting topic in interstellar chemistry. The study of possible reactions between detected species can help to understand the evolution in complexity of the interstellar matter and also allows knowing the formation of new molecules which could be candidates to be detected. We focus our attention on two molecules detected in space, vinyl alcohol (CH2CHOH) and azanone (HNO).
Aims. We aim to carry out a theoretical study of the ion-molecule reaction between protonated vinyl alcohol and azanone. The viability of formation of complex organic molecules (COMs) from these reactants is expected to provide some insight into the formation of prebiotic species through gas phase reactions.
Methods. The reaction of protonated vinyl alcohol with azanone has been theoretically studied by using ab initio methods. Stationary points on the potential energy surface (PES) were characterized at the second-order Moller-Plesset level in conjunction with the aug-cc-pVTZ (correlation-consistent polarized valence triple-zeta) basis set. In addition, the electronic energies were refined by means of single-point calculations at the CCSD(T) level (coupled cluster single and double excitation model augmented with a non-iterative treatment of triple excitations) with the same basis set.
Results. From a thermodynamic point of view, twelve products, composed of carbon, oxygen, nitrogen, and hydrogen which could be precursors in the formation of more complex biological molecules, can be obtained from this reaction. Among these, we focus especially on ionized glycine and two of its isomers. The analysis of the PES shows that only formation of cis- and trans-O-protonated imine acetaldehyde, CH2NHCOH+ and, CHNHCHOH+, are viable under interstellar conditions.
Conclusions. The reaction of protonated vinyl alcohol with azanone can evolve in the interstellar medium to more complex organic molecules of prebiotic interest. Our results suggest that imine acetaldehyde could be a feasible candidate molecule to be searched for in space.
Key words: astrobiology / astrochemistry / molecular processes / ISM: kinematics and dynamics / ISM: molecules / ISM: general
© ESO, 2017
1. Introduction
The availability of greater and greater accurate radio telescopes means that the number and variety of interstellar molecules detected increase continuously. Among the different sorts of detected molecules until today (about 180) especial interest is devoted to those organic species containing carbon, nitrogen, and oxygen atoms (denoted as complex organic molecules (COMs) if they contain carbon and have six of more atoms). These molecules are possible precursors of amino acids, as well as other prebiotic molecules which could play a crucial role in the interstellar synthesis of bio-molecules, the building blocks of life, under prebiotic conditions (Quan et al. 2016). How COMs are formed in the interstellar medium is a key issue for understanding the evolution to more complex bio-molecules (Rivilla et al. 2017). At present the models that try to explain the formation of COMs consider reactions on the surface of interstellar grains and gas-phase reactions (Garrod & Herbst 2006; Garrod et al. 2009; Vasyunin & Herbst 2013; Balucani et al. 2015).
Not only is interesting to know the formation processes of the detected species, another interesting subject is the possible evolution of these molecules. In this way, the study of possible reactions between detected species can help to understand the evolution in complexity of the interstellar matter and also allows knowing the formation of new molecules which could be candidates to be detected. In the present study we will focus on two interstellar species namely azanone and vinyl alcohol.
Nitroxyl or azanone, HNO, the second molecule with an N-O bond detected in the interstellar medium, was identified for the first time in 1977 toward Sgr B2 (OH) and NGC 2024 by Ulich et al. and subsequently confirmed by Hollis et al. (1991). In addition, HNO was found to be present in molecular clouds L134L, Sgr B2 (NW), DR 21 (OH), L134N, and W51M (Snyder et al. 1993; Ziurys et al. 1994). Three isomer with generic formula C2H4O, acetaldehyde, oxirane, and vinyl alcohol, have been detected in space (Gottlieb 1973; Dickens et al. 1997; Turner & Apponi 2001). The relative abundances of the isomers give information about their formation processes. In particular, vinyl alcohol CH2CHOH, the simplest enol, is known to be an important intermediate in many organic chemistry reactions. We will analyze the evolution possibilities in the interstellar medium of vinyl alcohol and azanone through the reaction between them. Gas-phase ion chemistry plays a crucial role in interstellar chemistry (Herbst 2001), and given the abundance of interstellar H3+, gas-phase protonation of HNO or CH2CHOH is feasible. On the other hand, proton affinity of vinyl alcohol is higher than that of azanone (Fairley et al. 1996; Grandinette et al.1992), therefore, we will study the ion-molecule reaction between azanone and protonated vinyl alcohol.
From these reactants we can obtain as products many COMs which are mainly composed from the elements basic of biomolecules, carbon, nitrogen, and/or oxygen. We note that one of the products that can be reached from this reaction is ionized glycine. It is well known that glycine, the simplest amino acid, is a key molecule in astrobiology. In spite of being identified in meteorites and comet, to date glycine has not been conclusively detected in the interstellar medium (Ceccarelli et al. 2000; Cunningham et al. 2007; Hollis et al. 2003a,b; Jones et al. 2007; Kuan et al. 2003; Snyder et al. 2005). A key aspect for the existence of glycine in the interstellar medium is to know if there are efficient routes of formation. In this regard, in recent years numerous theoretical and experimental studies have been devoted to the formation processes of glycine in the interstellar medium taking into account either gas phase or surface reactions (e.g., Andreazza et al. 2006; Barrientos et al. 2012; Basiuk & Kobayashi 2004; Blagojevic et al. 2003; Elsila et al. 2007; Largo et al. 2010a,b; Maeda & Ohno 2006; Nhlabatsi et al. 2016; Redondo et al. 2015; Rimola et al. 2012; Snow et al. 2007; Woon 2002). Another aspect of the non-detection of glycine to consider is that in general, the most abundant isomer of a given generic chemical formula observed in the ISM is the most stable one (minimum energy principle or MEP; Lattelais et al. 2011). Neutral glycine is not the most stable isomer and, therefore, probably not the most abundant one, which might explain why it has escaped detection so far.
The viability of formation of prebiotic molecules through the ion-molecule reaction involving protonated vinyl alcohol and azanone in space is analyzed in this study. The paper is organized as follows: in Sect. 2 we introduce the computational methods used in our study. In Sect. 3 we present firstly, a thermodynamic study of the possible products that can be reached from this process, and secondly, the potential energy surface of the reaction of protonated vinyl alcohol with azanone will be reported to analyze the viability under interstellar conditions of the different processes. In the final section we summarize the main findings of our study.
2. Computational methods
Reactants and products for the reaction between protonated vinyl alcohol and azanone were characterized employing ab initio methodologies. The stationary points on the [C2, H6, N, O2]+ singlet potential energy surface (PES; minima and transition states) connecting reactants with products are also located. Geometrical optimizations of the stationary points were performed at the second-order Moller-Plesset level (MP2; Moller & Plesset 1934) in conjunction with the basis set of Dunning aug-cc-pVTZ (correlation-consistent polarized valence triple-zeta including diffuse functions; Dunning 1989; Kendall et al. 1992). In the stationary points optimized harmonic vibrational frequencies were computed at the same level of theory. This calculation also allowed us to characterize the stationary points located on the potential energy surface, either a true minimum (all vibrational frequencies real) or a transition state (one of the frequencies, and just one, imaginary) and to estimate the zero-point vibrational energy correction (ZPE). For the transition states localized, intrinsic reaction coordinate (IRC) calculations (Gonzalez & Schelegel 1989, 1990) were carried out to check that they connect the desired processes.
Electronic energies over the MP2 optimized geometries were refined by performing single point calculations at the CCSD(T) (coupled-cluster single and double excitation model augmented with a non-iterative triple excitation correction) level (Raghavachari et al. 1989) employing the basis set of Dunning aug-cc-pVTZ. All valence electrons are included in the correlated calculations. In all cases the T1 diagnostic value for the CCSD(T) wave function was less than 0.03 suggesting that multiconfiguration effects were not significant. At the temperatures of the interstellar medium the entropic contributions to the Gibbs free energies of reactions are insignificants, so we will provide values of reaction energies. All results obtained in this work were computed by using codes implemented in Gaussian-09 program package (Frisch at al. 2010).
3. Results and discussion
Vinyl alcohol and azanone are present in the interstellar medium. In order to study their evolution into more complex molecules we will analyze the ion-molecule reaction between protonated vinyl alcohol and azanone. Protonation of a neutral molecule is a feasible process in interstellar proton-rich sources if its proton affinity (PA) is higher than that of the abundant species H2 or CO (101 and 142 kcal mol-1, respectively). PAs of both vinyl alcohol and azanone have been previously calculated at different levels of theory. A value of 171 kcal mol-1 for vinyl alcohol is reported by Fairley et al. (1996) at the G2 level; they also estimated an energy barrier of 41 kcal mol-1 for the isomerization process into the most stable isomer, protonated acetaldehyde. Later, Petrie (2005) reported PAs values for vinyl alcohol calculated at different theoretical levels which are included in the range 171–173 kcal mol-1. In addition, we checked dissociation of protonated vinyl alcohol, CH2CHOH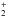 , into CH2CH+ and H2O. A value of 42.8 kcal mol-1 at the CCSD(T) level is obtained for dissociation energy showing that protonated vinyl alcohol is stable against dissociation into CH2CH+ and H2O. On the other hand, Grandinette et al. (1992) studied the protonation of HNO and the interconversion processes of various [H2,N,O]+ isomers at the G1 level. The most stable isomer of protonated HNO is obtained by protonation at the nitrogen atom, having a PA of 166 ± 2 kcal mol-1. Two stable isomers are obtained by protonation at the oxygen atom, trans-HNOH+ and cis-HNOH+, their reported PAs are 148 ± 2 and 141 ± 2 kcal mol-1, respectively. The computed isomerization barriers between the [H2, N, O]+ isomers are higher than 38 kcal mol-1.
, into CH2CH+ and H2O. A value of 42.8 kcal mol-1 at the CCSD(T) level is obtained for dissociation energy showing that protonated vinyl alcohol is stable against dissociation into CH2CH+ and H2O. On the other hand, Grandinette et al. (1992) studied the protonation of HNO and the interconversion processes of various [H2,N,O]+ isomers at the G1 level. The most stable isomer of protonated HNO is obtained by protonation at the nitrogen atom, having a PA of 166 ± 2 kcal mol-1. Two stable isomers are obtained by protonation at the oxygen atom, trans-HNOH+ and cis-HNOH+, their reported PAs are 148 ± 2 and 141 ± 2 kcal mol-1, respectively. The computed isomerization barriers between the [H2, N, O]+ isomers are higher than 38 kcal mol-1.
The values reported in the bibliography for PAs of vinyl alcohol and azanone are very similar to those calculated in this work. We obtain a value for the PA of vinyl alcohol of 172 kcal mol-1 and, a PA of 167 kcal mol-1 for protonation of azanone at the nitrogen atom. Both values are calculated at the CCSD(T) level. Given that the PA of vinyl alcohol is higher than that of azanone we have considered the ion-molecule reaction between CH2CHOH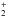 and HNO.
and HNO.
Relative (with respect to reactants) reaction enthalpies, in kcal mol-1, for the gas-phase reaction of protonated vinyl alcohol with azanone (CH2CHOH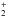 (1A) + HNO (1A′)) calculated at 0 K (ZPV energies included).
(1A) + HNO (1A′)) calculated at 0 K (ZPV energies included).
3.1. Thermodynamic of the reaction between protonated vinyl alcohol and azanone
Reaction enthalpies for the products which can be reached from the ion-molecule reaction between protonated vinyl alcohol and azanone are presented in this section. The results allow us to analyze the viability of their formation in space from a thermodynamic point of view. Table 1 summarizes the calculated reaction energies for different products obtained from this reaction. Their corresponding geometrical parameters, rotational constants and dipole moments are given in Fig. A.1 and Table A.1.
As seen from Table 1, four types of reaction have been considered in our study. Firstly, we have studied reactions corresponding to proton transfer processes (Products 1–3). Secondly, processes leading to a hydrogen atom elimination are taken into account (Products 4–6). Thirdly, we have considered reactions in which elimination of a hydrogen molecule takes place (Products 7 and 8). And finally, the reactions where a water molecule is eliminated will be addressed (Products 9 to 15).
Proton transfer processes to give protonated azanone, either on nitrogen atom or oxygen one, are endothermic paths (Products 1–3 in Table 1). At the CCSD(T) level, reaction enthalpies are 5.0 and 6.1 kcal mol-1 for protonation of azanone on nitrogen atom and 22.3 kcal mol-1 for oxygen protonation. These results are consistent with the relative values of PAs for the neutral species.
Ionized glycine, NH2CH2COOH+, and its two isomers namely, NH3CHCOOH+ and NH2CHC(OH)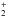 , can be reached from this reaction by hydrogen atom elimination. Formation of the three isomers are clearly exothermic processes (− 19.6, − 27.2 and − 39.6 kcal mol-1 at the CCSD(T) level, respectively) and the less favorable one from a thermodynamic point of view, is formation of ionized glycine (− 19.6 kcal mol-1). Simon et al. (2002) studied the stability and isomerization of glycine radical cation in gas phase and they found that interconversion processes between isomers involve net activation barriers. These barriers are estimated to be higher than 8.4 kcal mol-1 at the CCSD(T) level, therefore, the three isomers could be present in space if efficient synthesis paths exist.
, can be reached from this reaction by hydrogen atom elimination. Formation of the three isomers are clearly exothermic processes (− 19.6, − 27.2 and − 39.6 kcal mol-1 at the CCSD(T) level, respectively) and the less favorable one from a thermodynamic point of view, is formation of ionized glycine (− 19.6 kcal mol-1). Simon et al. (2002) studied the stability and isomerization of glycine radical cation in gas phase and they found that interconversion processes between isomers involve net activation barriers. These barriers are estimated to be higher than 8.4 kcal mol-1 at the CCSD(T) level, therefore, the three isomers could be present in space if efficient synthesis paths exist.
The reaction between CH2CHOH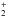 and HNO can also take place through elimination of molecular hydrogen. In this case we obtain as products N-protonated imine acetic acid, NH2CHCOOH+ (1A′), and a hydroxyl derivative of oxazetidine, c-NHCH2CO-OH+ (1A). Both processes are clearly exothermic (products are located 93.3 and 27.0 kcal mol-1 below reactants at the CCSD(T) level, respectively) and therefore, viable from a thermodynamic point of view. N-protonated imine acetic acid is an interesting prebiotic molecule; hydrogenation of the imide group could lead to protonated glycine. On the other hand, oxazetidine compounds could be relevant intermediates in the synthesis of biomolecules as it is shown by Pusterla & Bode (2015) obtaining serine from an oxazetidine amino acid.
and HNO can also take place through elimination of molecular hydrogen. In this case we obtain as products N-protonated imine acetic acid, NH2CHCOOH+ (1A′), and a hydroxyl derivative of oxazetidine, c-NHCH2CO-OH+ (1A). Both processes are clearly exothermic (products are located 93.3 and 27.0 kcal mol-1 below reactants at the CCSD(T) level, respectively) and therefore, viable from a thermodynamic point of view. N-protonated imine acetic acid is an interesting prebiotic molecule; hydrogenation of the imide group could lead to protonated glycine. On the other hand, oxazetidine compounds could be relevant intermediates in the synthesis of biomolecules as it is shown by Pusterla & Bode (2015) obtaining serine from an oxazetidine amino acid.
Different isomers of structural formula [C2, H4, N, O]+ can be reached from the reaction between protonated vinyl alcohol and azanone when a water molecule is eliminated. As can be seen in Table 1, all the products (c-CH2NHC- OH+, CH2NHCOH+, CHNHCHOH+, trans-NHCHCHOH+, cis-NHCHCHOH+, NH2CH2CO+ and NH2CHCOH+) are clearly exothermic targets. Isomer c-CH2NHC-OH+ (1A′) presents a three-member cycle and is an azaheterocyclic derived of aziridine, a potential prebiotic molecule, which in spite of being searched for in the interstellar medium no conclusive detection has been reported (Kuan et al. 2004). The corresponding open-chain isomer, CH2NHCOH+ (1A′), is located 10.5 kcal mol-1 above the aziridine derivative isomer at the CCSD(T) level. Hydrogen atom migration between the carbon atoms in CH2NHCOH+ gives the CHNHCHOH+ (1A′) isomer, which is found 19.7 kcal mol-1 less stable than isomer CH2NHCOH+. Interconversion processes between these isomers (c-CH2NHC-OH+, CH2NHCOH+, CHNHCHOH+) that present the same rearrangement between non-hydrogen atoms have been considered and the results are shown in Fig. 1 (panel a). The isomerization between the aziridine derivative isomer, c-CH2NHC-OH+, and the open-chain isomer, CH2NHCOH+, is not a direct process. It takes place through the c-CH2NHC-OH+ conformer which has the hydrogen atom bonded to the oxygen in position trans respect to the hydrogen bonded to carbon, denoted as c-CH2NHC-OH+-t. The interconversion process between these three member-ring conformers presents an energy barrier of 9.0 kcal mol-1 at the CCSD(T) level. The subsequent isomerization to the CH2NHCOH+ isomer involves the breaking of the C-C bond through transition state TS2a located 36.5 kcal mol-1 above c-CH2NHC-OH+-t isomer at the CCSD(T) level. Formation of the CHNHCHOH+ isomer from CH2NHCOH+ one through transition state TS3a implies an energy barrier of 42.2 kcal mol-1 at the CCSD(T) level. These important energy barriers indicate that the isomerization reactions between these isomers should have very slow rates. Thus, it is likely that c-CH2NHC-OH+, CH2NHCOH+ and, CHNHCHOH+ isomers could be present simultaneously in the interstellar medium.
 |
Fig. 1 Energy profile, in kcal/mol, for the isomerization of [C2, H4, N, O]+ isomers. Relative energies (including zero-point vibrational energies differences) are calculated respect to c-CH2NHC-OH+-t (panel a)) and trans-NHCHCHOH+ (panel b)) at the CCSD(T)/aug-cc-pVTZ//MP2/aug-cc-pVTZ level. |
Two conformers of O-protonated imine acetaldehyde, also with structural formula [C2,H4,N,O]+, are obtained as exothermic products from this reaction, which are denoted as trans-NHCHCHOH+ (1A′) and, cis-NHCHCHOH+ (1A′). Conformer trans-NHCHCHOH+ with the OH and NH groups in position trans is 8.3 kcal mol-1 more stable than the cis conformer, cis-NHCHCHOH+, at the CCSD(T) level. As can be seen in Fig. 1 (panel b) the conversion barrier for the cis conformer into the most stable trans is only of 0.4 kcal mol-1 (200 K) at the CCSD(T) level.
Finally, two isomers with the same N-C-C-O heavy atoms sequence are reached in the reaction of protonated vinyl alcohol with azanone when a water molecule is eliminated, namely NH2CH2CO+ (1A′) and, NH2CHCOH+ (1A). Both compounds have an amine and a carbonyl group. We note that we have also checked the products obtained by radical OH elimination, but all the paths located are endothermic and are not included in the present study.
We can conclude that, from a thermodynamic point of view twelve products can be obtained from this reaction under space conditions. All products are composed by carbon oxygen and nitrogen, and could be precursors of more complex biological molecules in the interstellar medium. Formation of NH2CHCOOH+ and, NH2CH2CO+ are the most favorable processes (reaction enthalpies are − 93.3 and − 81.3 kcal mol-1 at the CCSD(T) level, respectively).
3.2. Reaction of protonated vinyl alcohol and azanone: analysis of the PES
In this section we will analyze the [C2, H6, N, O2]+ singlet PES on which takes place the reaction between protonated vinyl alcohol and azanone. The stationary points, minima and transition states, on the PES connecting reactants with products are located. Given the complexity of the PES we have split the obtained results into three figures: Figs. 2–4.
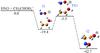 |
Fig. 2 Energy profile, in kcal mol-1, for the reaction of CH2CHOH |
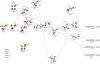 |
Fig. 3 Energy profile (continuation), in kcal mol-1, for the reaction of CH2CHOH |
 |
Fig. 4 Energy profile (continuation), in kcal mol-1, for the reaction of CH2CHOH |
In Fig. 2 we have collected the initial steps which are common for all the paths. The reaction of protonated vinyl alcohol with azanone starts with the formation of an ion-molecule complex I1 (1A) obtained through the interaction between one of the hydrogen atoms bonded to oxygen in protonated vinyl alcohol with the oxygen atom of azanone. Complex I1 is located 19.4 kcal mol-1 below the reactants at the CCSD(T) level. The formation of a nitrogen-carbon bond from intermediate I1 takes place through transition state TS1 (1A), where the simultaneous migration of a hydrogen atom from oxygen of CH2CHOH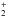 to oxygen of HNO and the formation of a N-C bond happen. Transition state TS1 is located 5.3 kcal mol-1 below the reactants making the process of formation of intermediate I2 (1A) (− 42.7 kcal mol-1 at the CCSD(T) level) viable under interstellar conditions. This initial pathway can be represented as:
to oxygen of HNO and the formation of a N-C bond happen. Transition state TS1 is located 5.3 kcal mol-1 below the reactants making the process of formation of intermediate I2 (1A) (− 42.7 kcal mol-1 at the CCSD(T) level) viable under interstellar conditions. This initial pathway can be represented as: 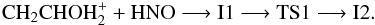 Once intermediate I2 is reached different possibilities of evolution are plausible and are shown in Fig. 3. Firstly, we consider the migration of a hydrogen atom from carbon to oxygen bonded to nitrogen and the subsequent elimination of a H2O molecule that leads to the cis conformer of O-protonated imine acetaldehyde, cis-NHCHCHOH+ (1A′) represented in Patha. This path involves transition state TS2 (1A) located − 17.2 kcal mol-1 respect to the reactants at the CCSD(T) level, an can be summarized as
Once intermediate I2 is reached different possibilities of evolution are plausible and are shown in Fig. 3. Firstly, we consider the migration of a hydrogen atom from carbon to oxygen bonded to nitrogen and the subsequent elimination of a H2O molecule that leads to the cis conformer of O-protonated imine acetaldehyde, cis-NHCHCHOH+ (1A′) represented in Patha. This path involves transition state TS2 (1A) located − 17.2 kcal mol-1 respect to the reactants at the CCSD(T) level, an can be summarized as 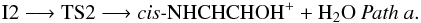 As can be seen in Fig. 3 formation process of the cis conformer of O-protonated imine acetaldehyde is exothermic (− 57.1 kcal mol-1) and barrier free and therefore viable under interstellar conditions.
As can be seen in Fig. 3 formation process of the cis conformer of O-protonated imine acetaldehyde is exothermic (− 57.1 kcal mol-1) and barrier free and therefore viable under interstellar conditions.
The trans conformer of O-protonated imine acetaldehyde, trans-NHCHCHOH+ (1A′), can be reached through Pathb that involves many steps. This path begins with the isomerization of intermediate I2 into its conformer I3 (1A) through transition state TS3 (1A). Next, intermediate I3 leads to the cyclic intermediate I4 (1A) by formation of a N-C bond, involving transition state TS4 (1A). Finally from intermediate I4 the hydrogen atom migration from carbon to oxygen and the subsequent water elimination in TS5 (1A) gives as product trans-NHCHCHOH+. All the transition states corresponding to Pathb are below the reactants (− 37.5, − 35.3 and − 17.3 kcal mol-1 at the CCSD(T) level, respectively) making the global process viable in the interstellar medium: ![\begin{eqnarray*} &&\rm I2 \longrightarrow TS3 \longrightarrow I3 \longrightarrow TS4 \longrightarrow I4 \longrightarrow TS5 \longrightarrow \\[-1.5mm] &&\quad\quad\rm \textit{trans-}NHCHCHOH^{+} + H_{2}O ~\textit{Path~ b}. \end{eqnarray*}](/articles/aa/full_html/2017/07/aa31186-17/aa31186-17-eq36.png) Other possible way to evolve intermediate I2 is its isomerization into the cyclic intermediate I5 (1A) (Pathsc.1 and c.2), a conformer of intermediate I4. The subsequent migration of the OH group from nitrogen to the carbon supporting the OH and the simultaneous rupture of the C-C bond leads to intermediate I6 (1A). From I6, the migration of a hydrogen atom from one of the carbon atoms to oxygen followed by a water molecule elimination gives as products CH2NHCOH+ and CHNHCHOH+, depending on the carbon from which the hydrogen migration occurs. These processes correspond to Pathsc.1 and c.2, respectively, in Fig. 3 and can be summarized as
Other possible way to evolve intermediate I2 is its isomerization into the cyclic intermediate I5 (1A) (Pathsc.1 and c.2), a conformer of intermediate I4. The subsequent migration of the OH group from nitrogen to the carbon supporting the OH and the simultaneous rupture of the C-C bond leads to intermediate I6 (1A). From I6, the migration of a hydrogen atom from one of the carbon atoms to oxygen followed by a water molecule elimination gives as products CH2NHCOH+ and CHNHCHOH+, depending on the carbon from which the hydrogen migration occurs. These processes correspond to Pathsc.1 and c.2, respectively, in Fig. 3 and can be summarized as 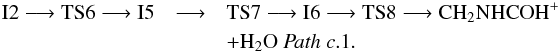
 The limiting step for Pathsc.1 and c.2 is the isomerization between intermediates I5 and I6 that involves transition state TS7 (1A) which lies only 1.1 kcal mol-1 below the reactants at the CCSD(T) level. The rest of transitions states are clearly more stable than the reactants. Therefore, formation of isomers CH2NHCOH+ and CHNHCHOH+ is viable under interstellar conditions.
The limiting step for Pathsc.1 and c.2 is the isomerization between intermediates I5 and I6 that involves transition state TS7 (1A) which lies only 1.1 kcal mol-1 below the reactants at the CCSD(T) level. The rest of transitions states are clearly more stable than the reactants. Therefore, formation of isomers CH2NHCOH+ and CHNHCHOH+ is viable under interstellar conditions.
Finally, from intermediate I2 the migration of the hydrogen atom bonded to oxygen to the nitrogen one and simultaneous formation of a carbon oxygen bond leads to the four-member cyclic intermediate I8 (1A) (Pathd). This isomerization takes place through transition state TS10 (1A) located clearly below the reactants (− 10.9 kcal mol-1 at the CCSD(T) level).
Once intermediate I8 is reached (Pathd) different paths are characterized and collected in Fig. 4 (Panel a and b). Isomer I8 can isomerize into O-protonated glycine, isomer I9 (1A), by hydrogen atom transfer from carbon to oxygen through transition state TS11 (1A) located 4.6 kcal mol-1 above the reactants at the CCSD(T) level (Fig. 4 (panel a), Pathd.1a). From I9, the direct elimination of a hydrogen atom in O-protonated glycine gives ionized glycine. Pathd.1a can be summarized as 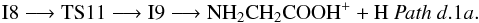 Also from I9, the hydrogen atom migration from the carbon to the adjacent nitrogen leads to intermediate I10 (1A) (isomer of protonated glycine) through transition state TS12 (1A) located − 56.4 kcal mol-1 below the reactants. The direct elimination of a hydrogen bonded to either an oxygen or a nitrogen atom in intermediate I10 gives two isomers of ionized glycine, NH3CHCOOH+ (Pathd.1b.1) or NH2CHC(OH)
Also from I9, the hydrogen atom migration from the carbon to the adjacent nitrogen leads to intermediate I10 (1A) (isomer of protonated glycine) through transition state TS12 (1A) located − 56.4 kcal mol-1 below the reactants. The direct elimination of a hydrogen bonded to either an oxygen or a nitrogen atom in intermediate I10 gives two isomers of ionized glycine, NH3CHCOOH+ (Pathd.1b.1) or NH2CHC(OH)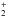 (Pathd.1b.2), these processes can be summarized as
(Pathd.1b.2), these processes can be summarized as 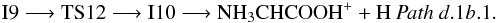
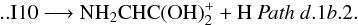 Finally, from I9, the most stable isomer of protonated glycine, NH3CH2COOH+ (1A) (isomer I12, located 128.2 kcal mol-1 below the reactants at the CCSD(T) level) can be obtained by going through its conformer I11 (1A) (Fig. 4 (panel a), Pathsd.1c.1a and d.1c.1b). Proton transfer from oxygen to nitrogen in intermediate I11 leads to I12 through transition state TS14 (1A) located − 92.3 kcal mol-1 at the CCSD(T) level. Once N-protonated glycine (intermediate I12) is obtained it can give ionized glycine (Pathd.1c.1a) and its isomer NH3CHCOOH+ (Pathd.1c.1b) by hydrogen atom elimination:
Finally, from I9, the most stable isomer of protonated glycine, NH3CH2COOH+ (1A) (isomer I12, located 128.2 kcal mol-1 below the reactants at the CCSD(T) level) can be obtained by going through its conformer I11 (1A) (Fig. 4 (panel a), Pathsd.1c.1a and d.1c.1b). Proton transfer from oxygen to nitrogen in intermediate I11 leads to I12 through transition state TS14 (1A) located − 92.3 kcal mol-1 at the CCSD(T) level. Once N-protonated glycine (intermediate I12) is obtained it can give ionized glycine (Pathd.1c.1a) and its isomer NH3CHCOOH+ (Pathd.1c.1b) by hydrogen atom elimination: 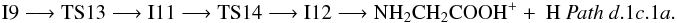
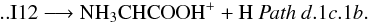 From N-protonated glycine we can also reach N-protonated imine acetic acid through transition state TS15 (1A) (− 41.9 kcal mol-1 at the CCSD(T) level):
From N-protonated glycine we can also reach N-protonated imine acetic acid through transition state TS15 (1A) (− 41.9 kcal mol-1 at the CCSD(T) level): 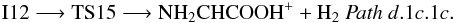 Intermediate I11 can also evolve by hydrogen atom migration from nitrogen to oxygen leading to intermediate I13 (1A) through transition state TS16 (1A) located 49.4 kcal mol-1 below the reactants at the CCSD(T) level (Fig. 4 (panel a), Pathd.1c.2). Intermediate I13 is an ion-molecule complex that directly dissociates into H2O and the derived of aziridine, c-CH2NHC-OH+. The process can be summarized as
Intermediate I11 can also evolve by hydrogen atom migration from nitrogen to oxygen leading to intermediate I13 (1A) through transition state TS16 (1A) located 49.4 kcal mol-1 below the reactants at the CCSD(T) level (Fig. 4 (panel a), Pathd.1c.2). Intermediate I13 is an ion-molecule complex that directly dissociates into H2O and the derived of aziridine, c-CH2NHC-OH+. The process can be summarized as 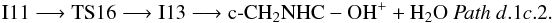 Hydrogen transfer from nitrogen to oxygen can also take place in intermediate I10. In this case, the loss of a water molecule to give as product NH2CHCOH+ takes place through the ion molecule complex I15 (1A). The process, Pathd.1d (Fig. 4, panel b), involves transition states TS17 (1A) and TS18 (1A) (− 68.3 and − 64.8 kcal mol-1 at the CCSD(T) level, respectively) and it can be summarized as:
Hydrogen transfer from nitrogen to oxygen can also take place in intermediate I10. In this case, the loss of a water molecule to give as product NH2CHCOH+ takes place through the ion molecule complex I15 (1A). The process, Pathd.1d (Fig. 4, panel b), involves transition states TS17 (1A) and TS18 (1A) (− 68.3 and − 64.8 kcal mol-1 at the CCSD(T) level, respectively) and it can be summarized as: 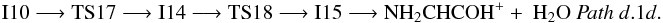 Another possible evolution path for intermediate I9 is the elimination of a water molecule by hydrogen atom migration between oxygen atoms through transition state TS19 (1A) (located 57.6 kcal mol-1 below the reactants at the CCSD(T) level) that gives as product NH2CH2CO+, Pathd.1e in Fig. 4, panel b:
Another possible evolution path for intermediate I9 is the elimination of a water molecule by hydrogen atom migration between oxygen atoms through transition state TS19 (1A) (located 57.6 kcal mol-1 below the reactants at the CCSD(T) level) that gives as product NH2CH2CO+, Pathd.1e in Fig. 4, panel b: 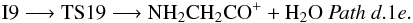 Finally, the cyclic intermediate I8 can also lead to the hydroxyl derivative of oxazetidine, c-CH2NHOC-OH+, by elimination of a hydrogen molecule (see Pathd.2 in Fig. 4, panel b). This process involves transition state TS20 (1A′) located 8.8 kcal mol-1 above the reactants at the CCSD(T) level. The global process, even though being exothermic, presents a net activation barrier (≈4400 K) and therefore is not viable under interstellar conditions;
Finally, the cyclic intermediate I8 can also lead to the hydroxyl derivative of oxazetidine, c-CH2NHOC-OH+, by elimination of a hydrogen molecule (see Pathd.2 in Fig. 4, panel b). This process involves transition state TS20 (1A′) located 8.8 kcal mol-1 above the reactants at the CCSD(T) level. The global process, even though being exothermic, presents a net activation barrier (≈4400 K) and therefore is not viable under interstellar conditions;  The results obtained for the evolution of isomer I8 presented in Fig. 4 (panels a and b) show that once O-protonated glycine, intermediate I9, is reached, many exothermic products, such as ionized glycine NH2CH2COOH+ (Pathsd.1a and d.1c.1a), its isomers, NH3CHCOOH+ (Pathsd.1b.1 and d.1c.1b) and NH2CHC(OH)
The results obtained for the evolution of isomer I8 presented in Fig. 4 (panels a and b) show that once O-protonated glycine, intermediate I9, is reached, many exothermic products, such as ionized glycine NH2CH2COOH+ (Pathsd.1a and d.1c.1a), its isomers, NH3CHCOOH+ (Pathsd.1b.1 and d.1c.1b) and NH2CHC(OH)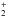 (Pathd.1b.2), or derived of aziridine, c-CH2NHC-OH+ (Pathd.1c.2), are available. However, the formation of O-protonated glycine, NH2CH2C(OH)
(Pathd.1b.2), or derived of aziridine, c-CH2NHC-OH+ (Pathd.1c.2), are available. However, the formation of O-protonated glycine, NH2CH2C(OH)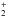 (I9), takes place through transition state TS11 located ≈2300 K above the reactants. Therefore, the evolution of intermediate I8 into the products shown in Fig. 4 is not viable under interstellar conditions.
(I9), takes place through transition state TS11 located ≈2300 K above the reactants. Therefore, the evolution of intermediate I8 into the products shown in Fig. 4 is not viable under interstellar conditions.
From the analysis of the potential energy surface of the reaction of protonated vinyl alcohol and azanone (Figs. 2–4) we can notice that even if formation of twelve prebiotic molecules (composed of C, N, O, and H) are exothermic processes, only Pathsa, b, c.1 and, c.2 giving cis- and trans-O-protonated imine acetaldehyde, CH2NHCOH+ and CHNHCHOH+, respectively, are barrier free. As can be seen in Fig. 3, the formation processes of both cis and trans conformers of O-protonated imine acetaldehyde are the most favorable from both a thermodynamic (largest exothermicity) and a kinetic (lowest energy barrier) point of view. As discussed in the previous section, the trans conformer of O-protonated imine acetaldehyde is 8.3 kcal mol-1 more stable than the cis one at the CCSD(T) level (see Table 1) and the conversion barrier from cis conformer into the most stable trans is only of 0.4 kcal mol-1 (≈200 K) at the CCSD(T) level (Fig. 1). Therefore,formation of conformer trans of O-protonated imine acetaldehyde is the most favorable product for the reaction between protonated vinyl alcohol and azanone.
O-protonated imine acetaldehyde is a direct precursor of neutral imine acetaldehyde, NHCHCOH (1A′), that is an isomer of the recently detected methyl isocyanate (Halfen et al. 2015; Cernicharo et al. 2016). It should be pointed out that in general, the most stable ion may not give necessarily the most stable neutral isomer (Lattelais et al. 2009; Karton & Talbi 2014; Loomis et al. 2015).
4. Conclusions
A theoretical study of the viability of formation of prebiotic molecules through the ion-molecule reaction involving protonated vinyl alcohol and azanone in space is carried out. Vinyl alcohol and azanone are present in the interstellar medium and their PAs, 172 kcal mol-1 and, 167 kcal mol-1 at the CCSD(T) level, respectively, suggest that both isomers should react quite easily in proton-rich interstellar media to give the protonated species. As PA of vinyl alcohol is higher than that of azanone we have studied the ion-molecule reaction between protonated vinyl alcohol and azanone.
Firstly we have reported a thermodynamic analysis of the products that can be reached from this reaction by elimination of hydrogen atom, molecule, and H2O. From a thermodynamic point of view twelve products can be obtained from this reaction under space conditions, among which we can stand out ionized glycine, NH2CH2COOH+, and two of its isomers, NH3CHCOOH+ and NH2CHC(OH)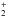 . All products are composed of carbon, oxygen, and nitrogen, and could be precursors in the formation of more complex biological molecules in the interstellar medium. Formation of NH2CHCOOH+ and, NH2CH2CO+ are the most favorable species (reaction enthalpies are − 93.3 and − 81.3 kcal mol-1 at the CCSD(T) level, respectively). In addition, spectroscopic parameters are reported for the exothermic products.
. All products are composed of carbon, oxygen, and nitrogen, and could be precursors in the formation of more complex biological molecules in the interstellar medium. Formation of NH2CHCOOH+ and, NH2CH2CO+ are the most favorable species (reaction enthalpies are − 93.3 and − 81.3 kcal mol-1 at the CCSD(T) level, respectively). In addition, spectroscopic parameters are reported for the exothermic products.
To explore the viability of formation of these products in the interstellar medium and their possible role in the formation of complex organic molecules in space a detailed study of the PES is reported. From the analysis of the PES we can conclude that formation of both cis and trans-O-protonated imine acetaldehyde, CH2NHCOH+ and CHNHCHOH+, are the only barrier free paths. Among them, the processes giving both cis and trans conformers of O-protonated imine acetaldehyde, are the most favorable from both a thermodynamic (− 57.1 and − 60.8 kcal mol-1 at the CCSD(T) level, respectively) and a kinetic point of view. The trans conformer is 8.3 kcal mol-1 more stable than the corresponding cis at the CCSD(T) level and the conversion barrier from the cis conformer into the most stable trans is only of 0.4 kcal mol-1 (≈200 K) at the CCSD(T) level. We could conclude that formation of conformer trans-O-protonated imine acetaldehyde would be the most favorable product for the reaction between protonated vinyl alcohol and azanone. Protonated COMs are not detected in ISM, it seems that they could evolve to neutral species by dissociative recombination. Therefore, imine acetaldehyde might be a candidate molecule to be searched for in the interstellar medium.
Acknowledgments
This work is a result of the collaboration of the COST Action TD 1308.
References
- Andreazza, H. J., Fitzgerald, M., & Bowie, J. H. 2006, Org. Biomol. Chem., 4, 2466 [CrossRef] [Google Scholar]
- Barrientos, C., Redondo, P., Largo, L., et al. 2012, ApJ, 748, 99 [NASA ADS] [CrossRef] [Google Scholar]
- Balucani, N., Ceccarelli, C., & Taquet, V. 2015, MNRAS, 449, L16 [Google Scholar]
- Basiuk, V. A., & Kobayashi, Y. 2004, Int. J. Quantum Chem., 99, 92 [CrossRef] [Google Scholar]
- Blagojevic, V., Petrie, S., & Bohme, D. K. 2003, MNRAS, 339, L7 [NASA ADS] [CrossRef] [Google Scholar]
- Ceccarelli, C., Loinard, L., Castets, A., et al. 2000, A&A, 362, 1122 [NASA ADS] [Google Scholar]
- Cernicharo, J., Kisiel, Z., Tercero, B., et al. 2016, A&A, 587, L4 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cunningham, M. R., Jones, P. A., Godfrey, P. D., et al. 2007, MNRAS, 376, 1201 [NASA ADS] [CrossRef] [Google Scholar]
- Dickens, J. E., Irvine, W. M., Ohishi, M., et al. 1997, ApJ, 489, 753 [NASA ADS] [CrossRef] [Google Scholar]
- Dunning, T. H. 1989, J. Chem. Phys., 90, 1007 [NASA ADS] [CrossRef] [Google Scholar]
- Elsila, J. E., Dworkin, J. P., Bernstein, M. P., Martin, M. P., & Sandford, S. A. 2007, ApJ, 660, 911 [NASA ADS] [CrossRef] [Google Scholar]
- Fairley, D. A., Scott, G. B. I., Freeman, C. G., Maclagan, R. G. A. R., & McEwan, M. J. 1996, J. Chem. Soc. Faraday Trans., 92, 1305 [CrossRef] [Google Scholar]
- Frisch, M. J., Trucks, G. W., Schlegel, H. B., et al. 2010, Gaussian 09, Gaussian Inc., Wallingford CT [Google Scholar]
- Garrod, R. T., & Herbst, E. 2006, A&A, 457, 927 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Garrod, R. T., Vasyunin, A. I., Semenov, D. A., et al. 2009, ApJ, 700, L43 [Google Scholar]
- Gonzalez, C., & Schelegel, H. B. 1989, J. Chem. Phys., 90, 2154 [NASA ADS] [CrossRef] [Google Scholar]
- Gonzalez, C., & Schelegel, H. B. 1990, J. Phys. Chem., 94, 5523 [CrossRef] [Google Scholar]
- Gottlieb, C. A. 1973, in Molecules in the Galactic Environment, eds. M. A. Gordon, & L. E. Snyder (Wiley-Interscience), 181 [Google Scholar]
- Grandinetti, F., Hrusak, J., Schröder, D., & Schwarz, H. 1992, J. Phys. Chem., 96, 2100 [CrossRef] [Google Scholar]
- Halfen, D. T., Ilyushin, V. V., & Ziurys, L. M. 2015, ApJ, 812, L5 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Herbst, E. 2001, Chem. Soc. Rev., 30, 168 [CrossRef] [MathSciNet] [Google Scholar]
- Hollis, J. M., Snyder, L. E., Ziurys, L. M., & McGonagle, D. 1991, in Atoms, Ions and Molecules: New Results in Spectral Line Astrophysics, eds. A. D. Haschick, & P. T. P. Ho, APS Conf. Ser., 1, 6, 407 [Google Scholar]
- Hollis, J. M., Pedelty, J. A., Boboltz, D. A., et al. 2003a, ApJ, 596, L235 [NASA ADS] [CrossRef] [Google Scholar]
- Hollis, J. M., Pedelty, J. A., Snyder, L. E., et al. 2003b, ApJ, 588, 353 [NASA ADS] [CrossRef] [Google Scholar]
- Jones, P. A., Cunningham, M. R., Godfrey, P. D., & Cragg, D. M. 2007, MNRAS, 374, 579 [NASA ADS] [CrossRef] [Google Scholar]
- Karton, A., & Talbi, D. 2014, Chem. Phys., 436, 22 [Google Scholar]
- Kendall, R. A., Dunning, T. H., & Harrison, R. J. 1992, J. Chem. Phys., 96, 6796 [NASA ADS] [CrossRef] [Google Scholar]
- Kuan, Y.-J., Charnley, S. B., Huang, H-C., et al. 2003, ApJ, 593, 848 [NASA ADS] [CrossRef] [Google Scholar]
- Kuan, J.-Y., Charnley, S. B., Huang, H.-C., et al. 2004, Adv. Space Res., 33, 31 [NASA ADS] [CrossRef] [Google Scholar]
- Largo, L., Redondo, P., Rayón, V. M., et al. 2010a, A&A, 516, A79 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Largo, L., Barrientos, C., Rayón, V. M., et al. 2010b, Int. J. Mass Spectrom., 295, 21 [CrossRef] [Google Scholar]
- Lattelais, M., Pauzat, F., Ellinger, Y., & Ceccarelli, C. 2011a, ApJ, 693, L133 [Google Scholar]
- Lattelais, M., Pauzat, F., Pilme, J., Ellinger, Y., & Ceccarelli, C. 2011b, A&A, 532, A39 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Loomis, R. A., McGuire, B. A., Shingledecker, C., et al. 2015, ApJ, 799, 34 [Google Scholar]
- Maeda, S., & Ohno, K. 2006, ApJ, 640, 823 [NASA ADS] [CrossRef] [Google Scholar]
- Moller, C., & Plesset, M. 1934, Ph.Rv. 46, 618 [Google Scholar]
- Nhlabatsi, Z. P., Bhasi, P., & Sitha, S. 2016, Phys. Chem. Chem. Phys., 18, 20109 [CrossRef] [Google Scholar]
- Petrie, S. 2005, J. Phys. Chem. A, 109, 6326 [CrossRef] [Google Scholar]
- Pusterla, I., & Bode, J. W. 2015, Nat. Chem., 7, 668 [CrossRef] [Google Scholar]
- Quan, D., Herbst, E., Corby, J. F., Durr, A., & Hassel, G. 2016, ApJ, 824, 129 [NASA ADS] [CrossRef] [Google Scholar]
- Raghavachari, K., Trucks, G. W., Pople, J. A., & Head-Gordon, M. 1989, Chem. Phys. Lett., 157, 479 [NASA ADS] [CrossRef] [Google Scholar]
- Redondo, P., Barrientos, C., & Largo, A. 2015, A&A, 579, A125 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Rimola, A., Sodupe, M., & Ugliengo, P. 2012, ApJ, 754, 24 [NASA ADS] [CrossRef] [Google Scholar]
- Rivilla, V. M., Beltrán, M. T., Cesaroni, R., et al. 2017, A&A, 598, A59 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Simon, S., Sodupe, M., & Bertran, J. 2002, J. Phys. Chem. A, 106, 5697 [CrossRef] [Google Scholar]
- Snow, J. L., Orlova, G., Blagojevic, V., & Bohme, D. K. 2007, J. Am. Chem. Soc., 129, 9910 [CrossRef] [PubMed] [Google Scholar]
- Snyder, L. E., Kuan, Y. J., Ziurys, L. M., & Hollis, J. M. 1993, ApJ, 403, L17 [NASA ADS] [CrossRef] [Google Scholar]
- Snyder, L. E., Lovas, F. J., Hollis, J. M., et al. 2005, ApJ, 619, 914 [NASA ADS] [CrossRef] [Google Scholar]
- Turner, B. E., & Apponi, A. J. 2001, ApJ, 561, L207 [NASA ADS] [CrossRef] [Google Scholar]
- Ulich, B. L, Hollis, J. M., & Snyder, L. E. 1977, ApJ, 217, L105 [NASA ADS] [CrossRef] [Google Scholar]
- Vasyunin, A. I., & Herbst, E. 2013, ApJ, 769, 34 [Google Scholar]
- Woon, D. E. 2002, ApJ, 571, L177 [NASA ADS] [CrossRef] [Google Scholar]
- Ziurys, L. M., Hollis, J. M., & Snyder, L. E. 1994, ApJ, 430, 706 [NASA ADS] [CrossRef] [Google Scholar]
Appendix A: Additional figure and table
In this appendix, we show the rotational constants and dipole moments of the products obtained from the reaction of protonated vinyl alcohol with azanone (Table A.1). In addition, their corresponding geometrical parameters are collected in Fig. A.1. Dipole moments were calculated at the center of mass.
Equilibrium rotational constants (MHz), and dipole moment (Debye) for the exothermic products obtained by the gas-phase reaction of protonated vinyl alcohol with azanone (CH2CHOH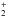 (1A) + HNO (1A′)) at the MP2/aug-cc-pVTZ level.
(1A) + HNO (1A′)) at the MP2/aug-cc-pVTZ level.
 |
Fig. A.1 MP2/aug-cc-pVTZ optimized geometries for the products obtained in the reaction of the protonated vinyl alcohol with azanone. Distances are given in angstroms and angles in degrees. |
All Tables
Relative (with respect to reactants) reaction enthalpies, in kcal mol-1, for the gas-phase reaction of protonated vinyl alcohol with azanone (CH2CHOH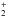 (1A) + HNO (1A′)) calculated at 0 K (ZPV energies included).
(1A) + HNO (1A′)) calculated at 0 K (ZPV energies included).
Equilibrium rotational constants (MHz), and dipole moment (Debye) for the exothermic products obtained by the gas-phase reaction of protonated vinyl alcohol with azanone (CH2CHOH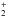 (1A) + HNO (1A′)) at the MP2/aug-cc-pVTZ level.
(1A) + HNO (1A′)) at the MP2/aug-cc-pVTZ level.
All Figures
 |
Fig. 1 Energy profile, in kcal/mol, for the isomerization of [C2, H4, N, O]+ isomers. Relative energies (including zero-point vibrational energies differences) are calculated respect to c-CH2NHC-OH+-t (panel a)) and trans-NHCHCHOH+ (panel b)) at the CCSD(T)/aug-cc-pVTZ//MP2/aug-cc-pVTZ level. |
| In the text | |
 |
Fig. 2 Energy profile, in kcal mol-1, for the reaction of CH2CHOH |
| In the text | |
 |
Fig. 3 Energy profile (continuation), in kcal mol-1, for the reaction of CH2CHOH |
| In the text | |
 |
Fig. 4 Energy profile (continuation), in kcal mol-1, for the reaction of CH2CHOH |
| In the text | |
 |
Fig. A.1 MP2/aug-cc-pVTZ optimized geometries for the products obtained in the reaction of the protonated vinyl alcohol with azanone. Distances are given in angstroms and angles in degrees. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


