Fig. C.3
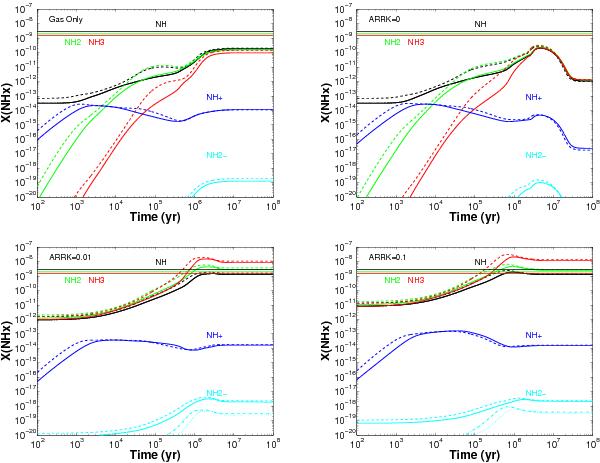
Translucent gas conditions in all models (see Table 3). Upper left: pure gas phase chemistry.
Upper right: gas and surface chemistry and inactive non-thermal
desorption efficiency aRRK = 0. Lower
left: gas and surface chemistry and active non-thermal desorption with
the typical efficiency aRRK = 0.01. Lower
right: gas and surface chemistry and high active non-thermal desorption
(aRRK =
0.1). The observed abundances and upper limits are indicated
with solid and dashed horizontal lines, respectively, following the respective
species colour code. The dot-dashed and dotted lines for
NH represent the estimated X(NH
represent the estimated X(NH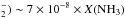 for the TK
= 30 K and 50 K models. If the reactive desorption mechanism is
active with the typical aRRK = 0.01, each NH,
NH2 and
NH3
species that is formed on the grain through a hydrogenation reaction has a
probability of 9.3 ×
10-3, 7.6
× 10-3, and 5.2 × 10-3, respectively,
to desorb into the gas phase. There it will become available for detection and for
follow-up reactions. Experiments by Dulieu et al.
(2013) indicate that this type of non-thermal desorption could be much
more efficient on bare grains than aRRK = 0.01. As shown, our
models are not very sensitive to the exact value of the desorption probability,
since the model with aRRK = 0.1 gives very similar
results to the model using aRRK = 0.01.
for the TK
= 30 K and 50 K models. If the reactive desorption mechanism is
active with the typical aRRK = 0.01, each NH,
NH2 and
NH3
species that is formed on the grain through a hydrogenation reaction has a
probability of 9.3 ×
10-3, 7.6
× 10-3, and 5.2 × 10-3, respectively,
to desorb into the gas phase. There it will become available for detection and for
follow-up reactions. Experiments by Dulieu et al.
(2013) indicate that this type of non-thermal desorption could be much
more efficient on bare grains than aRRK = 0.01. As shown, our
models are not very sensitive to the exact value of the desorption probability,
since the model with aRRK = 0.1 gives very similar
results to the model using aRRK = 0.01.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.






