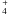| Issue |
A&A
Volume 549, January 2013
|
|
|---|---|---|
| Article Number | A93 | |
| Number of page(s) | 7 | |
| Section | Interstellar and circumstellar matter | |
| DOI | https://doi.org/10.1051/0004-6361/201219779 | |
| Published online | 03 January 2013 | |
Hydroxyacetonitrile (HOCH2CN) as a precursor for formylcyanide (CHOCN), ketenimine (CH2CNH), and cyanogen (NCCN) in astrophysical conditions
1
Aix-Marseille Univ, CNRS, PIIM UMR 7345, 13397
Marseille, France
e-mail: gregoire.danger@univ-amu.fr
2
Institut des Sciences Chimiques de Rennes, École Nationale
Supérieure de Chimie de Rennes, CNRS, UMR 6226, Avenue du Général Leclerc, CS 50837,
35708
Rennes Cedex 7,
France
Received: 8 June 2012
Accepted: 19 November 2012
Context. The reactivity in astrophysical environments can be investigated in the laboratory through experimental simulations, which provide understanding of the formation of specific molecules detected in the solid phase or in the gas phase of these environments. In this context, the most complex molecules are generally suggested to form at the surface of interstellar grains and to be released into the gas phase through thermal or non-thermal desorption, where they can be detected through rotational spectroscopy. Here, we focus our experiments on the photochemistry of hydroxyacetonitrile (HOCH2CN), whose formation has been shown to compete with aminomethanol (NH2CH2OH), a glycine precursor, through the Strecker synthesis.
Aims. We present the first experimental investigation of the ultraviolet (UV) photochemistry of hydroxyacetonitrile (HOCH2CN) as a pure solid or diluted in water ice.
Methods. We used Fourier transform infrared (FT-IR) spectroscopy to characterize photoproducts of hydroxyacetonitrile (HOCH2CN) and to determine the different photodegradation pathways of this compound. To improve the photoproduct identifications, irradiations of hydroxyacetonitrile 14N and 15N isotopologues were performed, coupled with theoretical calculations.
Results. We demonstrate that the photochemistry of pure hydroxyacetonitrile (HOCH2CN) (σphoto = 5.7 ± 1.0 × 10-20 photon s-1 cm2) under the influence of UV photons, or diluted in water ice (σphoto = 8.6 ± 1.0 × 10-20 photon s-1 cm2), leads to the formation of formylcyanide (CHOCN), ketenimine (CH2CNH), formaldehyde (CH2O), hydrogen cyanide (HCN), carbon monoxyde (CO), and carbon dioxyde (CO2); the presence of water increases its photodegradation rate. Furthermore, because hydroxyacetonitrile is more highly refractory than water, our results suggest that in astrophysical environments, hydroxyacetonitrile can be formed on icy grains from formaldehyde and hydrogen cyanide, and can be subsequently photodegradated in the water ice, or irradiated as a pure solid at the surface of dry grains after water desorption. As some of the hydroxyacetonitrile photochemistry products are detected in protostellar cores (e.g. formylcyanide or ketenimine), this compound maybe considered as one of the possible sources of these molecules at the grain surface in fairly cold regions. These photoproducts can then be released in the gas phase in a warmer region.
Key words: astrochemistry / methods: laboratory / molecular processes / ISM: molecules
© ESO, 2013
1. Introduction
To detect complex molecules in objects of the solar system, one possibility is in situ analysis, such as the Rosetta mission (Biele & Ulamec 2008; Gulkis & Alexander 2008), or sample return such as the Stardust mission (Brownlee et al. 2003). These missions provide interesting information about the formation of the organic matter in interplanetary environments. For instance, the Stardust mission sample return led to the first detection of glycine (NH2CH2COOH) in exogenous environments (Elsila et al. 2009). However, to detect molecules outside the solar system, only telescope observations are available. More than 160 molecules have been detected in the gas phase (Herbst & van Dishoeck 2009) of these environments through rotational spectroscopy. Among them, the aminoacetonitrile (NH2CH2CN) (Fig. 1), a potential precursor of glycine, has been detected within the Sagittarius B2 star nursery (Belloche et al. 2008). The detection of molecules inside the solid phase is obtained in the infrared range. The most abundant molecules detected on icy grains are simple, for instance methanol (CH3OH), carbon monoxide (CO), carbon dioxide (CO2), ammonia (NH3), formaldehyde (CH2O), or formic acid (HCOOH) (Dartois 2005). The detection of less abundant molecules in interstellar ice from infrared spectra is difficult since they are hidden by strong bands of water and/or silicates (Herbst & van Dishoeck 2009). However, complex molecules formed in the icy grains can be detected indirectly in the gas phase, since they could be sublimated in cold regions through photodesorption (Fayolle et al. 2011; Greenberg 1973), or in warmer regions (Garrod et al. 2008; Noble et al. 2012a). An exchange of molecules always occurs between the gas phase and the solid phase in interstellar environments (Ceccarelli 2008; Garrod et al. 2008). The importance of the grain-surface chemistry is highlighted by the modelling of the gas phase reaction, which underestimates the molecular abundances observed in the gas phase.
Therefore, the formation of complex molecules is mainly suggested to occur at the surface of interstellar grains (Fraser et al. 2002; Herbst & van Dishoeck 2009; Watanabe & Kouchi 2008; de Marcellus et al. 2011; Caro et al. 2002). For instance, this scenario is one of the possibilities to explain the formation of NH2CH2CN detected in the gas phase. NH2CH2CN has indeed been experimentally shown to form in the condensed phase through the photochemistry of acetonitrile and ammonia ice mixture (Danger et al. 2011b), or from the Strecker reaction that occurs at the grain surface (Danger et al. 2011a). However, its relatively low amount compared to the most dominant water molecule does not allow it to be detected on icy grains (Borget et al. 2012). Therefore, it is suggested that the subsequent warming of the grain causes the release of NH2CH2CN in the gas phase, which makes it detectable (Belloche et al. 2008; Borget et al. 2012).
Following this scenario, we investigated the possibility of using hydroxyacetonitrile (HOCH2CN) as a source of interstellar molecules already detected in the gas phase. HOCH2CN can be formed on the grain surface through the condensation of cyanide (CN−) and formaldehyde (CH2O) (Danger et al. 2012). This reaction competes with the first step of the Strecker reaction, which leads to NH2CH2CN formation (Fig. 1) (Danger et al. 2012), the first step of which is the aminomethanol formation (NH2CH2OH). The low-energy barrier difference between these two reactions suggests that if the Strecker scenario is accepted for the formation of a part of the aminoacetonitrile detected in the gas phase, HOCH2CN has to be considered as an astrophysically relevant compound that could be present in the solid phase in the same environments in which NH2CH2OH can be formed and detected (Fig. 1) (Bossa et al. 2009). Following this assumption, we study here the HOCH2CN photodegradation under VUV irradiation. Our results demonstrate that HOCH2CN is an interesting precursor of various molecules that are detected in the gas phase of hot cores. Experimental simulations indeed show that through its photodegradation, in dry or water ices, HOCH2CN could be a precursor of formylcyanide (CHOCN) (Remijan et al. 2008) and ketenimine (CH2CNH) (Lovas et al. 2006), which are detected in the gas phase of various astrophysical objects. Furthermore, because hydroxyacetonitrile is refractory compared to water, its photochemistry can occur in cold regions where water ices are dominant, or in warmer regions where water has sublimated.
 |
Fig. 1 Experimental pathways in astrophysical conditions for the hydroxyacetonitrile
formation via the direct reaction of ammonium cyanide with formaldehyde (ammonium
cyanide [NH |
2. Experimental
2.1. Operating systems
Hydroxyacetonitrile, also named glycolonitrile, was synthesized following Gaudry (1955), using KC14N for 14N-hydroxyacetonitrile, or KC15N for 15N-hydroxyacetonitrile. Hydroxyacetonitrile is directly deposited on the cold surface from its synthesis tube. Therefore, the amount deposited can only be estimated from the infrared spectra. For its irradiation in a water ice, hydroxyacetonitrile is co-deposited with water on the cold surface. Compounds were deposited on a gold-plated surface kept at 40 K with a model 21 CTI cold head. The thickness of the deposited solid films, assuming a density of 0.92 g cm-3 for H2O, is estimated to be around 0.1 μm, which is consistent with the interstellar ice mantle thickness. The 0.1–1 micron laboratory ices can be considered as optically thin at wavelengths longer than 160 nm (Cottin et al. 2003). However, this is not true for Lyman alpha photons. Therefore, the reported cross sections are upper limits when considering thinner ices, since Lyman alpha does contribute significantly to the measured UV flux used to calculate cross sections.
The warming-up of the samples was performed at a 5 K min-1 heating rate using a resistive heater along with a Lakeshore model 331 temperature controller. The infrared spectra of the sample were recorded in a reflection mode between 4000 and 600 cm-1 using a Nicolet Magna 750 FTIR spectrometer with an MCT detector. Each spectrum was averaged over one hundred scans with a 1 cm-1 resolution. The UV radiations (λ > 120 nm) are generated from a microwave discharge hydrogen flow lamp (Opthos instruments) separated from the vacuum chamber by a magnesium fluoride window. Lamp settings during irradiation are PH2 = 300 × 10-3 mbar, microwave forward power 70%, reflected power less than 10%. The distance between the lamp and the ice sample was about 10 cm. The flux was calibrated with the actinometry method decribed by Cottin et al. (2003) using the photolysis of methanol. The flux of the hydrogen lamp is 1.8 ± 0.6 × 1015 photons cm-2 s-1. Uncertainties on kinetic constant and photodissociation cross-sections were estimated from three different experiments with a confidence level of 95% (student value of 4.303 for three experimental values), and they therefore represent the experimental uncertainties.
2.2. Infrared spectroscopy for product attributions and quantifications
To corroborate compound attributions using literature data, vibrational analyses were performed to compute the harmonic vibrational frequencies for the 14N and 15N isotopologues (HOCH2CN, NCCN, CHOCN, HCN, NH=C=CH2). Nevertheless, to simplify this analysis, we did not take into account the effect of environment on the calculations. Calculations were performed using the Gaussian 98 package (Lee et al. 1988; Frisch et al. 1998) at the B3LYP/6-31G** level, which is known to supply reliable predictions of vibrational wavenumbers (Duvernay et al. 2004, 2007).
We used the band integration strengths to monitor the decrease in the reactant infrared
band intensities because they are consumed during the reaction, and to estimate how much
of each product is formed. Because of the reflecting mode, the calculated amount of
molecules obtained from the band integration was corrected for by a factor 2 since the
infrared beam is orthogonal to the sample holder. The amount of formaldehyde was obtained
from the band at 1717 cm-1 (which has a band strength
of 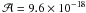 cm molecule-1,
Schutte et al. 1993), or the band
at 1498 cm-1 (
cm molecule-1,
Schutte et al. 1993), or the band
at 1498 cm-1 (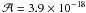 cm molecule-1,
Schutte et al. 1993). The amount of ketenimine
NH=C=CH2 was obtained with the band at 2035 cm-1
(
cm molecule-1,
Schutte et al. 1993). The amount of ketenimine
NH=C=CH2 was obtained with the band at 2035 cm-1
(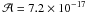 cm molecule-1,
Jacox & Milligan 1963; Hudson & Moore 2004). For the carbon dioxyde CO2 amount,
the band at 2340 cm-1 was used with a band strength
of
cm molecule-1,
Jacox & Milligan 1963; Hudson & Moore 2004). For the carbon dioxyde CO2 amount,
the band at 2340 cm-1 was used with a band strength
of 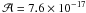 cm molecule-1
(Gerakines et al. 1995). Since the band strength
of the nitrile stretching mode is unknown, for the formyl cyanide CHOCN, the carbonyl
stretching mode of carbonyl was used. Its band strength was approximated
to
cm molecule-1
(Gerakines et al. 1995). Since the band strength
of the nitrile stretching mode is unknown, for the formyl cyanide CHOCN, the carbonyl
stretching mode of carbonyl was used. Its band strength was approximated
to 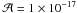 cm molecule-1,
which is a common value used for carbonyl stretching mode. The amount of carbon monoxyde
was obtained from the band at 2136 cm-1
(
cm molecule-1,
which is a common value used for carbonyl stretching mode. The amount of carbon monoxyde
was obtained from the band at 2136 cm-1
(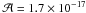 cm molecule-1,
Gerakines et al. 1995). For the hydrogen cyanide
HCN, the CN stretching mode was used with a band strength
of
cm molecule-1,
Gerakines et al. 1995). For the hydrogen cyanide
HCN, the CN stretching mode was used with a band strength
of 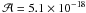 cm molecule-1
(Hudson & Moore 2004). The nitrile stretching
band of acetonitrile grows below the nitrile stretching mode of the hydroxyacetonitrile.
The acetonitrile amount was thus obtained by estimating the contribution of
hydroxyacetonitrile degradation to the 2265 cm-1 band using the
hydroxyacetonitrile band at 1050 cm-1. The amount of acetonitrile was then
estimated by substracting the amount of photodegradated hydroxyacetonitrile to the band
at 2265 cm-1.
cm molecule-1
(Hudson & Moore 2004). The nitrile stretching
band of acetonitrile grows below the nitrile stretching mode of the hydroxyacetonitrile.
The acetonitrile amount was thus obtained by estimating the contribution of
hydroxyacetonitrile degradation to the 2265 cm-1 band using the
hydroxyacetonitrile band at 1050 cm-1. The amount of acetonitrile was then
estimated by substracting the amount of photodegradated hydroxyacetonitrile to the band
at 2265 cm-1.
 |
Fig. 2 Pure hydroxyacetonitrile deposited at 40 K directly from its tube synthesis. |
3. Results and discussion
3.1. Photodegradation of pure hydroxyacetonitrile (HOCH2CN)
In a previous publication (Danger et al. 2012), we have demonstrated that the hydroxyacetonitrile can be formed in laboratory ice experiments, which simulate the reactions that could occur at the surface of interstellar grains (Fig. 1). In astrophysical environments, molecules can be submitted to several physical processes that could alter them (Throop 2011), such as UV processing. In this section, we are interested in investigating the stability and evolution of the hydroxyacetonitrile under UV irradiation at 40 K. Pure 14N-hydroxyacetonitrile was deposited directly from its tube synthesis at 40 K. The corresponding infra-red spectrum is displayed in Fig. 2, while the experimental band positions for the isotopologues 14N and 15N are collected in Table 1, along with their vibrational assignments. We also include in this table the theoretical frequency shifts Δν(14N-15N) between the 14N and the 15N hydroxyacetonitrile obtained from infra-red spectra and those calculated using the B3LYP/6-31G** level of calculation. The modes that imply nitrogen motion will be the most affected by the isotopic substitution. For example, the stronger calculated frequency shift is obtained for the nitrile mode of 31 cm-1, in good agreement with the experimental value of 38 cm-1. We use throughout the frequency shift induced by isotopic substitution in the nitrile region to investigate the assignment of the photoproducts.
After 240 min of irradiation, around 50% of the hydroxyacetonitrile is consumed. The evolution of the absorbance of the band located at 1060 cm-1 as a function of the time is fitted by a first-order kinetic rate, giving a kinetic constant of 1.1 ± 0.2 × 10-4 s-1. Furthermore, because the photon flux of our lamp is 1.8 ± 0.6 × 1015 photon cm-2 s-1, the corresponding photodissociation cross-section for the hydroxyacetonitrile is estimated to be σphoto = 5.7 ± 1.0 × 10-20 photon-1 cm2. For pure 15N-hydroxyacetonitrile the kinetic constant is 1.1 ± 0.2 × 10-4 s-1, giving a photodissociation cross-section of σphoto = 5.8 ± 1.0 × 10-20 photon-1 cm2.
Positions and attributions of infrared absorption bands of pure 14N and 15N hydroxyacetonitrile (HOCH2CN) at 40 K.
 |
Fig. 3 Infrared spectrum of pure hydroxyacetonitrile after 240 min of VUV irradiations at 40 K A), and the difference between spectrum of A) with the one of hydroxyacetonitrile before irradiation in the range 2300–2200 cm-1 B). |
The infrared spectrum obtained after 240 min of VUV irradiation at 40 K shows the appearance of new infrared bands compared to the initial hydroxyacetonitrile (Table 1, and Fig. 2 vs. Fig. 3A). The majority of these new bands appears in the range 2000–2350 cm-1. The band at 2341 cm-1 is attributed to carbon dioxide (Gerakines et al. 1995), and the one at 2135 cm-1 to carbon monoxide (Gerakines et al. 1995). Bands at 2090 cm-1, 2168 cm-1, and 2235 cm-1 are attributed to CN stretching modes of the hydrogen cyanide complex (HCN) (Danger et al. 2011a; Hudson & Moore 2004; Noble et al. 2012b), cyanogen (NCCN) (Hudson & Moore 2004; Stroh et al. 1989), and formylcyanide (CHOCN) (Lewis-Bevan et al. 1992), respectively. The presence of CHOCN is strengthened by bands at 1697 cm-1, 1370 cm-1, and 905 cm-1 (Lewis-Bevan et al. 1992). The band at 2035 cm-1 is attributed to ketenimine (CH2CNH) (Hudson & Moore 2004; Jacox & Milligan 1963), which is confirmed by the band at 1125 cm-1 (Hudson & Moore 2004; Jacox & Milligan 1963). The two bands at 1720 cm-1 and 1498 cm-1 are attributed to the formation of formaldehyde (Schutte et al. 1993). Furthermore, the growth of water is also observed with the appearance of bands at 3300 cm-1 and 1663 cm-1 (Gerakines et al. 1995). The difference spectrum between the deposit and after the 240 min of irradiation shows that new bands also grow under the one of the nitrile stretchings of the hydroxyacetonitrile at 2260 cm-1 (Fig. 3B). One band at 2276 cm-1 is attributed to the 13C carbon dioxide (Gerakines et al. 1995), and another band at 2257 cm-1 is tentatively attributed to the nitrile band of acetonitrile (CH3CN) (d’Hendecourt & Allamandola 1986; Hudson & Moore 2004). Therefore, the photodegradation of hydroxyacetonitrile is efficient and leads to interesting molecules that are observed in the gas phase of interstellar objects, such as ketenimine (Lovas et al. 2006), formylcyanide (Remijan et al. 2008), formaldehyde, hydrogen cyanide, or acetonitrile (Remijan et al. 2004).
 |
Fig. 4 Evolution of the photo-products from the primary photodegradation pathways of HOCH2CN at 40 K A), and from secondary photodegradation pathways B). The arrow refers to the time of the measurement of the photoproducts from the primary photodegradation process of HOCH2CN. |
However, photoproduct assignments from infrared spectroscopy in solid state is far from obvious, especially when only one band is observed. Photolysis was performed using the 15N isotopologue of hydroxyacetonitrile to verify the previous 14N assignments. We compared the experimental frequency shifts Δν (14N-15N) in the nitrile region (2300–2000 cm-1) with the corresponding theoretical shifts (Table 2), since this region is the most affected by isotopic substitution on nitrogen atoms. We also compared experimental and theoretical frequency shifts in the nitrile region between the reactant 14N, used as a reference, and the 14N photoproducts (noted ΔνHOCH2C14N in Table 2). Consequently, a photoproduct attribution will be considered as relevant if the following conditions are fulfilled:
-
i its bands have to be observed from the 14N isotopologue;
-
ii its bands have to be observed from the 15N isotopologue;
-
iii the experimental and theoretical frequency shifts Δν(14N-15N) in the nitrile region have to be coherent;
-
iv the observed difference in frequency ΔνHOCH2C14N between hydroxyacetonitrile and its daughter products must be consistent with theory.
As shown in Table 2, the comparison between the theoretical and experimental frequency shifts confirms our previous assignments except for CH3CN. The calculated frequency shift between HOCH2CN and CH3CN is −11 cm-1, while we experimentally observe + 8 cm-1. Even if the Δν (14N-15N) is fairly coherent between experimental and theoretical data, these results cannot resolve the ambiguity of the presence of CH3CN assignment for the observed band at 2257 cm-1 with the 14N isotopologue (2236 cm-1 for 15N). The photodegradation of the 15N isotopologue confirms the CHOCN, NH=C=CH2, NCCN, and HCN photoproduct formation, while the CH3CN attribution remains ambiguous.
Positions and attributions of infrared absorption bands of photoproducts formed after the VUV irradiation of 14N or 15N hydroxyacetonitrile at 40 K during 240 min.
After attributing hydroxyacetonitrile photoproducts, we tentatively estimated the different reaction pathways for the hydroxyacetonitrile degradation under VUV irradiation. The bands of products formed during this photochemical process were integrated. By tracing the evolution of the column density as a function of the fluence, it is possible to distinguish the first photodegradation processes from the secondary ones (Fig. 4). The photoformation evolution fittings show a first-order exponential growth with kinetic formation rates of 1.4 × 10-2 s-1 for ketenimine, 8.8 × 10-3 s-1 for formylcyanide, and 1.5 × 10-2 s-1 for formaldehyde (Fig. 4A). These three photoproducts are therefore the first pathways of hydroxyacetonitrile photodegradation (Fig. 5). The branching ratio of the corresponding processes was estimated to be at 5 min of irradiation of 10% for ketenimine, 15% for formylcyanide, and 75% for formaldehyde. Due to the development of secondary photodegradation processes, the branching ratios evolve after 240 min of irradiation, giving 35% for ketenimine and acetonitrile, 25% for formylcyanide, and 39% for formaldehyde. Five photoformation evolutions concerning CO2, CO, HCN, NCCN, and CH3CN display a latency period that differs depending on the molecule (Fig. 4B). These formations are therefore considered as secondary photodegradation processes. Figure 6 displays proposed formation pathways of these molecules. The carbon monoxide (CO) could come from the photodegradation of formaldehyde (Eq. (2)), and of formylcyanide (Eq. (1)). The carbon dioxide (CO2) could be formed by reaction between CO and the hydroxyl radical (OH) (Eq. (4)). The dimerization of CN radical could lead to the cyanogen formation (NCCN, Eq. (3)). Finally, acetonitrile (CH3CN, Eq. (5)) could be formed by the tautomerization of ketenimine under VUV irradiation.
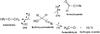 |
Fig. 5 Primary degradation pathways that occur during the VUV irradiation of pure hydroxyacetonitrile HOCH2CN. The branching ratios of each pathway are reported in brackets. |
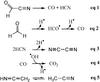 |
Fig. 6 Propositions for secondary degradation pathways, which occur during the VUV irradiation of pure hydroxyacetonitrile HOCH2CN. |
3.2. Photodegradation of HOCH2CN in water ice
 |
Fig. 7 UV irradiation of HOCH2CN diluted in a water ice (1:10 HOCH2CN:H2O). A) Infrared specta monitored after the H2O:HOCH2CN ice formation at 40 K, t0, and after 240 min of VUV irradiation at 40 K, t240. B) Infrared spectrum obtained at 190 K after the warming of the sample displayed in A) t240 with a 5 K min-1 temperature ramp. Note: vibration mode: stretching (ν), bending (δ), rocking (ρ), torsion (τ), wagging (ω). Type of vibration mode: asymmetric (as), symmetric (s). |
Water is one of the most abundant molecules that constitutes the icy mantle of
interstellar grains in cold regions. In this section, to enhance the astrophysical
implication of this work, we describe HOCH2C14N dilution in water
ice (1:10 HOCH2CN:H2O). The ratio was estimated from the
experimental spectrum by using the band strength of HOCH2C14N
at 1057 cm-1 (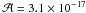 cm molecule-1,
Danger et al. 2012), and the one of water
at 3243 cm-1 (
cm molecule-1,
Danger et al. 2012), and the one of water
at 3243 cm-1 (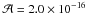 cm
molecule-1, Gerakines et al. 1995). In
a water environment, infrared bands present small shifts relative to pure
HOCH2C14N, i.e. the band at 2060 cm-1 for a pure
deposit shifts to 2063 cm-1 in a water ice. The evolution under UV irradiation
of the absorbance of the band located at 1057 cm-1 (1050 cm-1 for
pure HOCH2C14N) as a function of the time was fitted by a
first-order kinetic rate, giving a kinetic constant
of 1.7 ± 0.2 × 10-4 s-1. Furthermore, because the photon flux of
our lamp is 1.8 ± 0.6 × 1015 photon cm-2 s-1, the
corresponding photodissociation cross-section for the hydroxyacetonitrile is estimated to
be σphoto = 8.6 ± 1.0 × 10-20 photon-1 cm2.
In presence of water, the disappearance of HOCH2C14N is thus 50%
higher than the pure ice, which implies that a water environment increases the
photodegradation rate of this product. The infrared monitoring of the irradiation shows
that after 240 min of irradiation, the same infrared bands appear as are observed during
the irradiation of the pure solid (Fig. 7A vs.
Fig. 3A) with small frequency shifts due to the
water environment. The bands are observed at 2340 cm-1 for CO2,
at 2276 cm-1 for 13CO2, at 2236 cm-1
for CHOCN, at 2169 cm-1 for NCCN, at 2136 cm-1 for CO,
at 2088 cm-1 for HCN, at 2038 cm-1 for HN=C=CH2, and
at 1715 cm-1 for CH2O.
cm
molecule-1, Gerakines et al. 1995). In
a water environment, infrared bands present small shifts relative to pure
HOCH2C14N, i.e. the band at 2060 cm-1 for a pure
deposit shifts to 2063 cm-1 in a water ice. The evolution under UV irradiation
of the absorbance of the band located at 1057 cm-1 (1050 cm-1 for
pure HOCH2C14N) as a function of the time was fitted by a
first-order kinetic rate, giving a kinetic constant
of 1.7 ± 0.2 × 10-4 s-1. Furthermore, because the photon flux of
our lamp is 1.8 ± 0.6 × 1015 photon cm-2 s-1, the
corresponding photodissociation cross-section for the hydroxyacetonitrile is estimated to
be σphoto = 8.6 ± 1.0 × 10-20 photon-1 cm2.
In presence of water, the disappearance of HOCH2C14N is thus 50%
higher than the pure ice, which implies that a water environment increases the
photodegradation rate of this product. The infrared monitoring of the irradiation shows
that after 240 min of irradiation, the same infrared bands appear as are observed during
the irradiation of the pure solid (Fig. 7A vs.
Fig. 3A) with small frequency shifts due to the
water environment. The bands are observed at 2340 cm-1 for CO2,
at 2276 cm-1 for 13CO2, at 2236 cm-1
for CHOCN, at 2169 cm-1 for NCCN, at 2136 cm-1 for CO,
at 2088 cm-1 for HCN, at 2038 cm-1 for HN=C=CH2, and
at 1715 cm-1 for CH2O.
The irradiated ice was then warmed from 40 K to 300 K with a temperature ramp of 5 K min-1. When the temperature is approaching 180 K, the water desorbs and drives the HOCH2C14N photoproducts out of the sample holder. However, after the water desorption, some HOCH2C14N remains on the sample holder (Fig. 7B). HOCH2C14N is effectively more refractory than water, since its desorption temperature in our laboratory conditions is around 210 K, while that of water is 180 K. Therefore, HOCH2C14N can still remain on the sample holder after the water desorption, which implies that HOCH2C14N initially diluted in a water ice can be photodegradated under UV irradiation either in water ice or as a pure solid, depending on the temperature in astrophysical environments.
4. Astrophysical implications
The detection of amino acids in various meteorites leads to a search for some chemical pathways that could explain their formation in these media. One possible pathway that is relevant for the synthesis of some of them is the Strecker synthesis (Fig. 1) (Lerner & Cooper 2005). The major hypothesis is that this reaction can occur inside asteroids or meteorites through a liquid phase reaction (Cronin et al. 1994; Burton et al. 2012). This reaction is also presented as one possible pathway for the formation of the aminoacetonitrile detected in the gas phase of hot cores. The various steps leading to the aminoacetonitrile can indeed occur at the surface or inside icy grains (Danger et al. 2011a, 2012). The subsequent warming-up of grains can lead to an aminoacetonitrile release in the gas phase, which could explain, at least partly, the detection of aminoacetonitrile in warm regions. When the Strecker reaction is performed using all the necessary reactants, i.e. formaldehyde, ammonia, formic acid, and hydrogen cyanide, concurrent reactions will occur. It was indeed shown that from an ice containing formaldehyde, ammonia, and hydrogen cyanide, the aminomethanol formation (Ea = 4.5 kJ mol-1, Bossa et al. 2009) competes with that of hydroxyacetonitrile (Ea = 3.9 kJ mol-1, Danger et al. 2012). At this time, the only available rotational spectrum of these molecules is that of aminoacetonitrile. Aminomethanol and hydroxyacetonitrile cannot be detected yet due to the lack of their respective rotational spectra. Therefore, it could be interesting to perform such an analysis for each compound.
In this contribution, considering that the aminoacetonitrile is detected in the gas phase (Belloche et al. 2008), that the aminomethanol is supposed to have band contributions in the W33A spectrum (Bossa et al. 2009), and that the hydroxyacetonitrile is formed under the same conditions as aminomethanol at the surface or inside icy grains (Danger et al. 2012), the presence of hydroxyacetonitrile in these objects is likely, and it should be available for subsequent photoreactivity. The hydroxyacetonitrile photodegradation was therefore investigated in the laboratory at 40 K through UV irradiation using an H2 discharge lamp with a photon flux of 2 × 1015 photons cm-2 s-1. Depending on the grain position with regard to the nearby star, molecules carried on these grains can be submitted to UV irradiations with flux varying from 0 to 108 photons cm-2 s-1 (Ciesla & Sandford 2012). After 240 min of irradiation, the UV dose is 3 × 1019 photons cm-2, which could correspond to a UV dose during the time life in a nebula disk (106 years) of a grain submitted to 106 photons cm-2 s-1. In our experimental conditions, we demonstrate that the hydroxyacetonitrile is photodegradated in three primary pathways that lead to the formation of ketenimine, formylcyanide, and formaldehyde, three molecules that have been detected in the gas phase of warm regions. These photodegradation pathways occur when hydroxyacetonitrile is diluted in a water ice (Fig. 7A) as well as when hydroxyacetonitrile is irradiated in its pure form (Fig. 3A). Furthermore, experiments performed in the presence of water showed that even after the water desorption, which drives out a large part of its photoproducts from the sample holder (Fig. 7B), hydroxyacetonitrile remains at the grain surface because it is more refractory than water. Therefore, hydroxyacetonitrile can be considered to be one of the sources of various astrophysical relevant molecules, such as formylcyanide (Remijan et al. 2008), or ketenimine (Lovas et al. 2006). Our results support the hypothesis that the formation of a large part of complex gas phase molecules detected in warm regions is mainly formed at the grain surface, as for the hydroxyacetonitrile formation or for the formation of its photoproducts, and once these molecules are formed, they can be released into the gas phase through their photodesorption or thermaldesorption (Fig. 7B). The importance of hydroxyacetonitrile for the formation of these molecular species should be estimated by modelling grain evolution (Vasyunina et al. 2012).
5. Conclusion
The Strecker synthesis is presented as one possible chemical pathway for the formation of the aminoacetonitrile NH2CH2CN in astrophysical environments. However, this reaction competes with various side reactions. One of these side reactions concerns the hydroxyacetonitrile HOCH2CN formation in competition with the aminomethanol NH2CH2OH. Taking into account this scenario, the hydroxyacetonitrile can thus be considered as a potential astrophysical molecule that should be searched for. We investigated the hydroxyacetonitrile stability under UV irradiations and showed that its photodegradation as a pure solid gives a photodissociation cross-section of σphoto = 5.7 ± 1.0 × 10-20 photon-1 cm2 for the 14N and 15N isotopologues. Its irradiation after dilution in water ice gives a photodissociation cross-section of σphoto = 8.6 ± 1.0 × 10-20 photon-1 cm2. The photodegradation of the hydroxyacetonitrile leads to the formation of several molecules detected in the gas phase of hot cores. Its primary photodegradation pathway indeed leads to the formation of ketenimine, formaldehyde, hydrogen cyanide, and formylcyanide. The secondary pathways lead to the formation of hydrogen cyanide, cyanogen, carbon monoxide, and carbon dioxide. Furthermore, since hydroxyacetonitrile is more highly refractory than water, its photodegradation can occur in water ice as well as at the surface of grains after the water desorption. During the grain cycle, grains can be warmed, which could provide the release into the gas phase of hydroxyacetonitrile photoproducts. Hydroxyacetonitrile can therefore be considered as a potential source of gas phase molecules detected in astrophysical environments.
Acknowledgments
This work was founded by the French national program Physique Chimie du Milieu Interstellaire (P.C.M.I), Environnements Planétaires et Origines de la Vie (EPOV) and the Centre National d’Études Spatiales (C.N.E.S).
References
- Belloche, A., Menten, K. M., Comito, C., et al. 2008, A&A, 482, 179 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Biele, J., & Ulamec, S. 2008, Space Sci. Rev., 138, 275 [Google Scholar]
- Borget, F., Danger, G., Duvernay, F., et al. 2012, A&A, 541, A114 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Bossa, J. B., Theule, P., Duvernay, F., & Chiavassa, T. 2009, ApJ, 707, 1524 [NASA ADS] [CrossRef] [Google Scholar]
- Brownlee, D., Tsou, P., Anderson, J., et al. 2003, J. Geophys. Res. Planets, 108 [Google Scholar]
- Burton, A. S., Stern, J. C., Elsila, J. E., Glavin, D. P., & Dworkin, J. P. 2012, Chem. Soc. Rev., 41, 5459 [CrossRef] [PubMed] [Google Scholar]
- Caro, G., Meierhenrich, U., Schutte, W., et al. 2002, Nature, 416, 403 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Ceccarelli, C. 2008, in IAU Symp., 251, 79 [Google Scholar]
- Ciesla, F. J., & Sandford, S. A. 2012, Science, 336, 452 [NASA ADS] [CrossRef] [Google Scholar]
- Cottin, H., Moore, M., & Benilan, Y. 2003, ApJ, 590, 874 [NASA ADS] [CrossRef] [Google Scholar]
- Cronin, J., Cooper, G., & Pizzarello, S. 1994, Adv. Space Res., 15, 91 [Google Scholar]
- Danger, G., Borget, F., Chomat, M., et al. 2011a, A&A, 535, A47 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Danger, G., Bossa, J. B., de Marcellus, P., et al. 2011b, A&A, 525, A30 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Danger, G., Duvernay, F., Theulé, P., Borget, F., & Chiavassa, T. 2012, ApJ, 756, 11 [NASA ADS] [CrossRef] [Google Scholar]
- Dartois, E. 2005, Space Sci. Rev., 119, 293 [NASA ADS] [CrossRef] [Google Scholar]
- de Marcellus, P., Meinert, C., Nuevo, M., et al. 2011, ApJ, 727 [Google Scholar]
- d’Hendecourt, L., & Allamandola, L. J. 1986, A&AS, 64, 453 [NASA ADS] [Google Scholar]
- Duvernay, F., Chiavassa, T., Borget, F., & Aycard, J. 2004, Chem. Phys., 298, 241 [NASA ADS] [CrossRef] [Google Scholar]
- Duvernay, F., Chatron-Michaud, P., Borget, F., Birney, D. M., & Chiavassa, T. 2007, Phys. Chem. Chem. Phys., 9, 1099 [CrossRef] [PubMed] [Google Scholar]
- Elsila, J. E., Glavin, D. P., & Dworkin, J. P. 2009, Meteor. Planet. Sci., 44, 1323 [NASA ADS] [CrossRef] [Google Scholar]
- Fayolle, E. C., Bertin, M., Romanzin, C., et al. 2011, ApJ, 739, L36 [NASA ADS] [CrossRef] [Google Scholar]
- Fraser, H., McCoustra, M., & Williams, D. 2002, Astron. Geophys., 43, 10 [CrossRef] [Google Scholar]
- Frisch, M., Trucks, G., Schlegel, H., et al. 1998, Gaussian 98, revision A8, 25 [Google Scholar]
- Garrod, R. T., Weaver, S. L. W., & Herbst, E. 2008, ApJ, 682, 283 [NASA ADS] [CrossRef] [Google Scholar]
- Gaudry, R. 1955, Organic Syntheses Coll., 3, 436 [Google Scholar]
- Gerakines, P., Schutte, W., Greenberg, J., & Vandishoeck, E. 1995, A&A, 296, 810 [NASA ADS] [Google Scholar]
- Greenberg, L. T. 1973, 52, 413 [Google Scholar]
- Gulkis, S., & Alexander, C. 2008, Space Sci. Rev., 138, 259 [NASA ADS] [CrossRef] [Google Scholar]
- Herbst, E., & van Dishoeck, E. F. 2009, ARA&A, 47, 427 [NASA ADS] [CrossRef] [Google Scholar]
- Hudson, R., & Moore, M. 2004, Icarus, 172, 466 [NASA ADS] [CrossRef] [Google Scholar]
- Jacox, M., & Milligan, D. E. 1963, J. Am. Chem. Soc., 85, 278 [CrossRef] [Google Scholar]
- Lee, C., Yang, W., & Parr, R. 1988, Phys. Rev., 37, 785 [Google Scholar]
- Lerner, N., & Cooper, G. 2005, Geochim. Cosmochim. Acta, 69, 2901 [NASA ADS] [CrossRef] [Google Scholar]
- Lewis-Bevan, W., Gaston, R., Tyrrell, J., Stork, W., & Salmon, G. 1992, J. Am. Chem. Soc., 114, 1933 [CrossRef] [Google Scholar]
- Lovas, F. J., Hollis, J. M., Remijan, A. J., & Jewell, P. R. 2006, ApJ, 645, 137 [Google Scholar]
- Noble, J., Congiu, E., Dulieu, F., & Fraser, H. J. 2012a, MNRAS, 421, 768 [NASA ADS] [Google Scholar]
- Noble, J., Theule, P., Borget, F., et al. 2012b, MNRAS, accepted [Google Scholar]
- Remijan, A., Sutton, E., Snyder, L., et al. 2004, ApJ, 606, 917 [NASA ADS] [CrossRef] [Google Scholar]
- Remijan, A. J., Hollis, J. M., Lovas, F. J., et al. 2008, ApJ, 675, 85 [Google Scholar]
- Schutte, W., Allamandola, L., & Sandford, S. 1993, Icarus, 104, 118 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Stroh, F., Winnewisser, B., Winnewisser, M., et al. 1989, Chem. Phys. Lett., 160, 105 [NASA ADS] [CrossRef] [Google Scholar]
- Throop, H. B. 2011, Icarus, 212, 885 [NASA ADS] [CrossRef] [Google Scholar]
- Vasyunina, T., Vasyunin, A. I., Herbst, E., & Linz, H. 2012, ApJ, 751, 105 [NASA ADS] [CrossRef] [Google Scholar]
- Watanabe, N., & Kouchi, A. 2008, Prog. Surf. Sci., 83, 439 [NASA ADS] [CrossRef] [Google Scholar]
All Tables
Positions and attributions of infrared absorption bands of pure 14N and 15N hydroxyacetonitrile (HOCH2CN) at 40 K.
Positions and attributions of infrared absorption bands of photoproducts formed after the VUV irradiation of 14N or 15N hydroxyacetonitrile at 40 K during 240 min.
All Figures
 |
Fig. 1 Experimental pathways in astrophysical conditions for the hydroxyacetonitrile
formation via the direct reaction of ammonium cyanide with formaldehyde (ammonium
cyanide [NH |
| In the text | |
 |
Fig. 2 Pure hydroxyacetonitrile deposited at 40 K directly from its tube synthesis. |
| In the text | |
 |
Fig. 3 Infrared spectrum of pure hydroxyacetonitrile after 240 min of VUV irradiations at 40 K A), and the difference between spectrum of A) with the one of hydroxyacetonitrile before irradiation in the range 2300–2200 cm-1 B). |
| In the text | |
 |
Fig. 4 Evolution of the photo-products from the primary photodegradation pathways of HOCH2CN at 40 K A), and from secondary photodegradation pathways B). The arrow refers to the time of the measurement of the photoproducts from the primary photodegradation process of HOCH2CN. |
| In the text | |
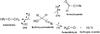 |
Fig. 5 Primary degradation pathways that occur during the VUV irradiation of pure hydroxyacetonitrile HOCH2CN. The branching ratios of each pathway are reported in brackets. |
| In the text | |
 |
Fig. 6 Propositions for secondary degradation pathways, which occur during the VUV irradiation of pure hydroxyacetonitrile HOCH2CN. |
| In the text | |
 |
Fig. 7 UV irradiation of HOCH2CN diluted in a water ice (1:10 HOCH2CN:H2O). A) Infrared specta monitored after the H2O:HOCH2CN ice formation at 40 K, t0, and after 240 min of VUV irradiation at 40 K, t240. B) Infrared spectrum obtained at 190 K after the warming of the sample displayed in A) t240 with a 5 K min-1 temperature ramp. Note: vibration mode: stretching (ν), bending (δ), rocking (ρ), torsion (τ), wagging (ω). Type of vibration mode: asymmetric (as), symmetric (s). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.