Table 3
TDDFT electronic excitation energies (in eV) of the complexes calculated with B3LYP/cc-pVTZ and ωB97/cc-pVTZ.
| B3LYP | ωB97 | |||||||||||
|
|
|
|||||||||||
| Figure | Molecule | E1 | E2 | E3 | E4 | E5 | E1 | E2 | E3 | E4 | E5 | |
|
|
||||||||||||
| Fig. 1.A | C2H4 + HCNH+ → [C2H4:HCNH]+ | 3.81 | 3.86 | 5.64 | 5.73 | 7.14 | 5.56 | 5.73 | 7.37 | 7.98 | 8.17 | |
|
Oscillator strength | 0.002 | 0.00 | 0.00 | 0.00 | 0.086 | 0.014 | 0.00 | 0.00 | 0.093 | 0.199 | |
| Fig. 1.B | C2H4+HNCH+ → [C2H4:HNCH]+ | 4.84 | 4.91 | 6.41 | 6.59 | 7.92 | 6.57 | 6.84 | 7.62 | 8.14 | 8.27 | |
|
Oscillator strength | 0.004 | 0.00 | 0.00 | 0.00 | 0.00 | 0.037 | 0.00 | 0.00 | 0.00 | 0.235 | |
| Fig. 1.C | C2H4+HCNH+ → [C2H4:HCNH]+, Boat | 6.71 | 7.41 | 7.61 | 7.93 | 9.55 | 7.01 | 7.74 | 7.99 | 8.56 | 9.79 | |
|
Oscillator strength | 0.00 | 0.003 | 0.105 | 0.095 | 0.001 | 0.00 | 0.002 | 0.15 | 0.076 | 0.001 | |
| Fig. 1.D | C2H4+HCNH+ → [C2H4:HCNH]+, Bridge | 3.51 | 6.54 | 6.7 | 7.27 | 7.97 | 3.68 | 6.98 | 7.13 | 7.65 | 8.54 | |
|
Oscillator strength | 0.001 | 0.003 | 0.029 | 0.012 | 0.248 | 0.001 | 0.041 | 0.029 | 0.025 | 0.343 | |
| Fig. 2.A | C2H4+H2CNH → [C2H4:H2CNH2]+ → [C2H4:H2CNH2]+ |
4.62 | 5.86 | 6.97 | 7.37 | 7.62 | 5.32 | 7.28 | 7.61 | 8.04 | 8.44 | |
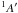
|
Oscillator strength | 0.309 | 0.00 | 0.023 | 0.139 | 0.003 | 0.314 | 0.00 | 0.245 | 0.00 | 0.001 | |
| Fig. 2.B | C2H4+H2CNH → [C2H4:H2CNH2]+, Boat → [C2H4:H2CNH2]+, Boat |
8.81 | 8.87 | 9.66 | 9.92 | 10.03 | 9.71 | 9.97 | 10.68 | 10.87 | 10.88 | |
| 1 A | Oscillator strength | 0.019 | 0.004 | 0.032 | 0.004 | 0.044 | 0.026 | 0.003 | 0.031 | 0.006 | 0.005 | |
| Fig. 3.A | HCCH+HCNH+ → [HCCH:HCNH]+ | 4.12 | 4.31 | 4.4 | 4.5 | 6.73 | 5.8 | 6.08 | 6.39 | 6.45 | 7.12 | |
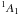
|
Oscillator strength | 0.00 | 0.016 | 0.001 | 0.00 | 0.00 | 0.00 | 0.002 | 0.00 | 0.014 | 0.00 | |
| Fig. 3.B | HCCH+HNCH+ → [HCCH:HNCH]+ | 4.89 | 5.1 | 5.34 | 5.45 | 6.74 | 6.19 | 6.81 | 7.39 | 7.44 | 7.51 | |
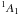
|
Oscillator strength | 0.00 | 0.014 | 0.001 | 0.00 | 0.00 | 0.00 | 0.001 | 0.012 | 0.00 | 0.00 | |
| Fig. 3.C | HCCH+HNCH+ → [HCCH:HNCH]+, Boat | 2.81 | 5.62 | 6.52 | 7.37 | 7.4 | 3.03 | 5.82 | 6.85 | 7.72 | 7.92 | |
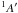
|
Oscillator strength | 0.005 | 0.00 | 0.00 | 0.00 | 0.027 | 0.005 | 0.00 | 0.00 | 0.00 | 0.035 | |
| Fig. 4 | HCN+C2H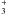 → [HCN:C2H3]+ → [HCN:C2H3]+ |
5.1 | 5.76 | 7.25 | 7.65 | 8.35 | 5.73 | 6.08 | 7.52 | 8.19 | 8.68 | |
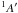
|
Oscillator strength | 0.00 | 0.3 | 0.00 | 0.00 | 0.11 | 0.00 | 0.327 | 0.00 | 0.00 | 0.264 | |
| Fig. 5.A | HCC+ + HCN → HCCHCN+, Branched | 3.06 | 3.33 | 4.9 | 5.34 | 6.39 | 3.17 | 3.39 | 5.6 | 6.04 | 7.69 | |
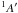
|
Oscillator strength | 0.00 | 0.007 | 0.011 | 0.021 | 0.365 | 0.00 | 0.009 | 0.016 | 0.177 | 0.299 | |
| Fig. 5.B | HCC+ + NCH → [HCCNCH]+, Linear | 4.32 | 4.46 | 4.47 | 7.46 | 8.74 | 4.46 | 4.66 | 4.67 | 7.89 | 8.67 | |
| 1Σ | Oscillator strength | 0.00 | 0.00 | 0.00 | 1.058 | 0.00 | 0.00 | 0.00 | 0.00 | 1.249 | 0.00 | |
| Fig. 6.A | HCCC+ + HCN → [HCCC(H)NCH]+, Branched | 1.62 | 1.7 | 4.32 | 4.39 | 5.11 | 1.66 | 2.26 | 5.08 | 6.41 | 6.44 | |
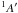
|
Oscillator strength | 0.00 | 0.003 | 0.006 | 0.001 | 0.00 | 0.00 | 0.006 | 0.187 | 0.104 | 0.00 | |
| Fig. 6.B | HCCC+ + HCN → [HCCCNCH]+, Linear | 0.83 | 3.21 | 4.32 | 4.62 | 5.37 | 0.89 | 3.44 | 4.54 | 5.47 | 5.74 | |
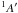
|
Oscillator strength | 0.001 | 0.001 | 0.028 | 0.002 | 0.315 | 0.001 | 0.001 | 0.062 | 0.003 | 0.343 | |
| Fig. 7 | H2CCH2+H2CNH+ → [H2CCH2:H2CNH]+ | 2.27 | 3.39 | 3.81 | 4.15 | 4.4 | 2.29 | 3.67 | 4.09 | 4.61 | 4.77 | |
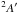
|
Oscillator strength | 0.23 | 0.00 | 0.001 | 0.003 | 1.00 | 0.21 | 0.002 | 0.003 | 0.001 | 0.01 | |
| Fig. 8.A | H2CNH2 + H2CNH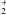 → [H2CNH2] → [H2CNH2]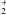 , Pi-stack , Pi-stack |
2.86 | 4.29 | 4.94 | 5.16 | 5.23 | 2.5 | 4.89 | 5.43 | 5.48 | 5.78 | |
|
Oscillator strength | 0.208 | 0.018 | 0.00 | 0.00 | 0.00 | 0.237 | 0.008 | 0.015 | 0.00 | 0.00 | |
| Fig. 8.B | H2CNH2 + H2CNH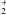 → [H2CNH2] → [H2CNH2] , Trans , Trans |
3.01 | 4.99 | 5.68 | 6.16 | 6.38 | 3.02 | 4.99 | 5.68 | 6.16 | 6.38 | |
|
Oscillator strength | 0.195 | 0.00 | 0.00 | 0.002 | 0.00 | 0.195 | 0.00 | 0.00 | 0.002 | 0.00 | |
Notes. Underneath the excitation energies the dimensionless oscillator strengths are presented. The electronic ground state is noted in the column with the figure name (the row below the figure name). 1 eV = 23.0605 kcal mol-1 = 1.16045 × 104 K.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


