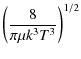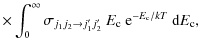| Issue |
A&A
Volume 517, July 2010
|
|
|---|---|---|
| Article Number | L2 | |
| Number of page(s) | 5 | |
| Section | Letters | |
| DOI | https://doi.org/10.1051/0004-6361/201015186 | |
| Published online | 30 July 2010 | |
LETTER TO THE EDITOR
Astronomical identification of CN-,
the smallest observed molecular anion![[*]](/icons/foot_motif.png) ,
,![[*]](/icons/foot_motif.png)
M. Agúndez1
- J. Cernicharo2 - M. Guélin3
- C. Kahane4 - E.
Roueff1 - J. K![]() os5 - F. J. Aoiz6
- F.
Lique7 - N. Marcelino2 -
J. R. Goicoechea2 - M. González García8
- C. A. Gottlieb9 - M. C.
McCarthy9 - P. Thaddeus9
os5 - F. J. Aoiz6
- F.
Lique7 - N. Marcelino2 -
J. R. Goicoechea2 - M. González García8
- C. A. Gottlieb9 - M. C.
McCarthy9 - P. Thaddeus9
1 - LUTH,
Observatoire de Paris-Meudon, 5 Place Jules Janssen, 92190 Meudon,
France
2 - Departamento de
Astrofísica, Centro de Astrobiología, CSIC-INTA, Ctra. de
Torrejón a Ajalvir km 4, 28850 Madrid, Spain
3 - Institut de
Radioastronomie Millimétrique, 300 rue de la Piscine, 38406
Saint Martin d'Héres, France
4 - Laboratoire d'Astrophysique de
l'Observatoire de Grenoble, 38041 Grenoble, France
5 - Department
of Chemistry and Biochemistry, University of Maryland, College
Park, MD 20742, USA
6 - Departamento de Química Física,
Facultad de Química, Universidad Complutense, 28040 Madrid,
Spain
7 - LOMC FRE 3102, CNRS Université du Havre, 25 rue
Philippe Lebon, BP 540, 76058 Le Havre, France
8 - Instituto de
Radioastronomía Milimétrica, Av Divina Pastora 7, Local 20,
18012 Granada, Spain
9 - Harvard-Smithsonian Center for
Astrophysics, 60 Garden Street, Cambridge, MA 02138, USA
Received 9 June 2010 / Accepted 5 July 2010
Abstract
We present the first astronomical detection of a diatomic
negative ion, the cyanide anion CN-, and quantum
mechanical
calculations of the excitation of this anion by means of
collisions with para-H2. The anion CN-
is identified by
observing the J = 2-1 and J =
3-2 rotational transitions in
the C-star envelope IRC +10216 with the IRAM 30-m telescope.
The
U-shaped line profiles indicate that CN-, like
the large anion
C6H-, is formed in the
outer regions of the envelope.
Chemical and excitation model calculations suggest that this
species forms from the reaction of large carbon anions with N
atoms, rather than from the radiative attachment of an electron to
CN, as is the case for large molecular anions. The unexpectedly
high abundance derived for CN-, 0.25% relative
to CN,
indicates that its detection in other astronomical sources is
likely. A parallel search for the small anion C2H-
remains
inconclusive, despite the previous tentative identification of the
J = 1-0 rotational transition. The
abundance of C2H- in
IRC +10216 is found to be vanishingly small, <0.0014%
relative to C2H.
Key words: astrochemistry - line: identification - molecular processes - stars: AGB and post-AGB - circumstellar matter - stars: individual: IRC +10216
1 Introduction
The molecular anions detected so far in the interstellar and circumstellar gas are all fairly heavy linear carbon chains consisting of three or more carbon atoms, and with neutral counterparts with large electron affinities: C4H-, C6H-, C8H-, C3N-, and C5N-(Cernicharo et al. 2007; Thaddeus et al. 2008; Cernicharo et al. 2008; Brünken et al. 2007a; Remijan et al. 2007; McCarthy et al. 2006). The abundance of these anions relative to the neutral counterparts increases with both size and the electron affinity of the neutral molecule, as expected for formation by radiative electron attachment (Herbst & Osamura 2008). On inspection, however, this process fails to explain the abundance of the shortest observed anions, in particular C4H- and C3N-. In IRC +10216, a carbon star envelope where both anions are found, C3N- has an anion-to-neutral abundance ratio about 50 times higher than that of C4H-, indicating that other formation processes may be at work (Thaddeus et al. 2008; Cernicharo et al. 2007; Agúndez 2009; Cordiner & Millar 2009). Studying the astronomical abundance of even shorter anions, in particular C2H- and CN-, whose formation by radiative electron attachment is very slow, should help us to answer this question.
In this Letter, we describe the identification in IRC +10216 of CN- and the results of a parallel search for C2H-. We also present quantum mechanical calculations of the collisional excitation of CN- by para-H2, using the calculated rate coefficients to model the observed lines. The chemistry of CN-in space is also briefly discussed.
2 Observations and identification of CN-
The C2H- and CN- anions are closed-shell molecules whose rotational spectrum has been recently measured in the laboratory (Amano 2008; Brünken et al. 2007b; Gottlieb et al. 2007). Their electric dipole moments are 3.1 and 0.65 Debye, respectively (Botschwina et al. 1995; Brünken et al. 2007b).
![\begin{figure}
\par\includegraphics[angle=0,width=7.5cm,clip]{15186fig1.eps}
\end{figure}](/articles/aa/full_html/2010/09/aa15186-10/Timg5.png)
|
Figure 1:
Spectra of IRC +10216 covering the J = 1-0
to J = 3-2transitions of CN-.
Grey horizontal boxes mark their expected
positions based on the laboratory frequencies and a linewidth of
29 km s-1. Shaded areas show
the fits to the line profiles
obtained with the CLASS method shell.
The high
spectral resolution spectrum of the J =
2-1 line shows the
expected position of the different hyperfine components with their
relative intrinsic strengths. The intensity scale is expressed as
|
| Open with DEXTER | |
Following our initial detection in IRC +10216 of a
![]() mK
line at the frequency of the J =
1-0 transition of C2H-
(Cernicharo et al. 2008),
we searched from January to April 2010
for the J = 2-1 transition
at 166.5 GHz. The line was not
detected with a
mK
line at the frequency of the J =
1-0 transition of C2H-
(Cernicharo et al. 2008),
we searched from January to April 2010
for the J = 2-1 transition
at 166.5 GHz. The line was not
detected with a ![]() rms noise level of 0.6 mK per 2 MHz
channel, casting doubt on the tentative identification of the
C2H- J
= 1-0 line.
rms noise level of 0.6 mK per 2 MHz
channel, casting doubt on the tentative identification of the
C2H- J
= 1-0 line.
The CN- observed lines are shown in
Fig. 1
and the derived line parameters are
given in Table 1. The
J = 3-2 transition of CN-
is shown in the top panel of
Fig. 1.
It appears as a U-shaped line with
the expected half width (
![]() km s-1)
that agrees in frequency to within 0.6 MHz with that of the CN-transition.
The J = 2-1 transition of CN-,
shown in the
middle panel of Fig. 1 with a
spectral
resolution of 2 MHz and of 320 kHz (2.7
and 0.4 km s-1respectively),
coincides with a broad spectral feature with a
complex shape that is unusual for IRC +10216, since it is
neither
U-shaped, flat-topped, nor parabolic. It is most accurately
described as a blend, as shown in Fig. 1,
that can be well fitted with two components, one U-shaped with a
half width
km s-1)
that agrees in frequency to within 0.6 MHz with that of the CN-transition.
The J = 2-1 transition of CN-,
shown in the
middle panel of Fig. 1 with a
spectral
resolution of 2 MHz and of 320 kHz (2.7
and 0.4 km s-1respectively),
coincides with a broad spectral feature with a
complex shape that is unusual for IRC +10216, since it is
neither
U-shaped, flat-topped, nor parabolic. It is most accurately
described as a blend, as shown in Fig. 1,
that can be well fitted with two components, one U-shaped with a
half width ![]() of 14.5 km s-1
centered on the
frequency of the J = 2-1 transition of CN-
(see
Table 1),
the other with a parabolic
profile, a half width
of 14.5 km s-1
centered on the
frequency of the J = 2-1 transition of CN-
(see
Table 1),
the other with a parabolic
profile, a half width ![]() of
of ![]() km s-1,
and
a rest frequency of 224
km s-1,
and
a rest frequency of 224
![]() MHz that is close to
that
of the 102,9-92,8
rotational transition of SiC2 in
the
MHz that is close to
that
of the 102,9-92,8
rotational transition of SiC2 in
the ![]() vibrational state (224 519.7 MHz; Izuha
et al. 1994).
Since other
vibrational state (224 519.7 MHz; Izuha
et al. 1994).
Since other ![]() lines of SiC2 with similar intrinsic
strengths have similar shapes, half widths (
lines of SiC2 with similar intrinsic
strengths have similar shapes, half widths (
![]() km s-1),
and intensities (
km s-1),
and intensities (
![]() mK) in our
mK) in our
![]() 0.9 mm
data (Kahane et al., in prep.) as our fitted
parabolic component, there is little doubt that this component
comes from SiC2. We note that the CN-
J = 2-1 transition
has several hyperfine components due to the nitrogen quadrupole,
which can be grouped into three blocks lying at 224 523.9,
224 525.1, and 224 527.2 MHz, with relative
line strengths of 0.27, 1,
and 0.12, respectively (Gottlieb
et al. 2007). Because of the severe
blending with the SiC2
0.9 mm
data (Kahane et al., in prep.) as our fitted
parabolic component, there is little doubt that this component
comes from SiC2. We note that the CN-
J = 2-1 transition
has several hyperfine components due to the nitrogen quadrupole,
which can be grouped into three blocks lying at 224 523.9,
224 525.1, and 224 527.2 MHz, with relative
line strengths of 0.27, 1,
and 0.12, respectively (Gottlieb
et al. 2007). Because of the severe
blending with the SiC2 ![]() and the limited sensitivity of
the astronomical observations, only the strongest hyperfine
component is clearly visible in the spectrum of IRC +10216,
while
the middle strength component is hidden between the two stronger
fitted lines (see Fig. 1), and
the weakest
hyperfine component lies below the noise level of the spectrum.
Finally, the bottom panel of Fig. 1 shows
the spectrum covering the CN- J
= 1-0 transition, which is
heavily blended with a strong line of C6H. The
limited spectral
resolution (1 MHz) and the broadening of this CN-
line by the
hyperfine structure (there are three components separated by
1-2 MHz; Gottlieb
et al. 2007) makes it difficult to determine the
relative
contributions of C6H and CN-
to the observed line.
and the limited sensitivity of
the astronomical observations, only the strongest hyperfine
component is clearly visible in the spectrum of IRC +10216,
while
the middle strength component is hidden between the two stronger
fitted lines (see Fig. 1), and
the weakest
hyperfine component lies below the noise level of the spectrum.
Finally, the bottom panel of Fig. 1 shows
the spectrum covering the CN- J
= 1-0 transition, which is
heavily blended with a strong line of C6H. The
limited spectral
resolution (1 MHz) and the broadening of this CN-
line by the
hyperfine structure (there are three components separated by
1-2 MHz; Gottlieb
et al. 2007) makes it difficult to determine the
relative
contributions of C6H and CN-
to the observed line.
Table 1: Observed line parameters of CN-.
There are no good candidates other than CN- for the carrier of the 336 777.0 MHz line. The only plausible molecule with a transition within 2 MHz of the observed frequency, according to the line catalogs of Cernicharo, CDMS (Müller et al. 2005), and JPL (Pickett et al. 1998), is 13CCH, whose NJ,F1,F = 47/2,4,7/2-3 7/2,4,7/2 transition lies at 336 775.7 MHz. This molecule, however, is ruled out since the nearby 4 7/2,4,9/2-3 7/2,4,9/2 transition at 336 756.2 MHz, with a slightly higher intrinsic strength, is not present in our data (see Fig. 1). Since no other plausible candidate can be found for the 224 525.4 MHz line and since unidentified lines of that intensity are rare in IRC +10216 at these frequencies, we conclude that we have almost certainly detected CN-. Confirmation of this identification would be highly desirable, but may not be easy to obtain. The next two rotational transitions of CN-, at 449 and 561 GHz, cannot be observed from the ground owing to high atmospheric opacity, and still higher J transitions may be too weak to detect in a cool source such as the outer envelope of IRC +10216.
The J = 3-2 line of CN-,
which appears free of contamination
by background lines, has a pronounced U-shaped profile, which for
a spherical expanding envelope indicates that the emission is more
extended than the half-power beam of the telescope (7'' at
336 GHz). Thus, CN- appears to be
confined to the same outer
envelope of IRC +10216 as are other molecular anions observed
in
this source (e.g. Cernicharo
et al. 2007; Thaddeus et al. 2008; Cernicharo
et al. 2008). A column density
of ![]() cm-2
and a rotation temperature of 16 K were derived from a
rotational diagram constructed with the
velocity integrated intensities of the J = 2-1
and 3-2 lines
given in Table 1,
based on the assumption
of a uniform source with a radius of 20'', which is typical of
molecules distributed in the outer shell. The rotation temperature
is consistent with CN- emission from the cool
outer envelope.
With a column density of the CN radical of
cm-2
and a rotation temperature of 16 K were derived from a
rotational diagram constructed with the
velocity integrated intensities of the J = 2-1
and 3-2 lines
given in Table 1,
based on the assumption
of a uniform source with a radius of 20'', which is typical of
molecules distributed in the outer shell. The rotation temperature
is consistent with CN- emission from the cool
outer envelope.
With a column density of the CN radical of
![]() cm-2,
derived from several hyperfine components of the N
=
1-0 and N = 3-2 transitions, we estimate a
CN-/CN abundance
ratio of 0.25%, which is comparable to the C3N-/C3N
ratio in this source (0.52%; Thaddeus
et al. 2008).
cm-2,
derived from several hyperfine components of the N
=
1-0 and N = 3-2 transitions, we estimate a
CN-/CN abundance
ratio of 0.25%, which is comparable to the C3N-/C3N
ratio in this source (0.52%; Thaddeus
et al. 2008).
From the upper limit to the J =
2-1 line of C2H-,
we
derive a 3![]() column density of <
column density of <
![]() cm-2,
based on the assumption of a source with a radius of 20'' and
a rotation temperature of 20 K. The estimated
C2H-/C2H
abundance ratio (<0.0014%) is at least 5 times
lower than the already small C4H-/C4H
ratio
(Agúndez 2009).
cm-2,
based on the assumption of a source with a radius of 20'' and
a rotation temperature of 20 K. The estimated
C2H-/C2H
abundance ratio (<0.0014%) is at least 5 times
lower than the already small C4H-/C4H
ratio
(Agúndez 2009).
3 Modeling and discussion
| Figure 2: Abundance distribution derived for CN- in the envelope of IRC +10216 (thick grey line labeled as ``CN- fit''), as it reproduces the CN- observed line profiles (see Fig. 3). Also shown are the abundances of CN-, CN, and other molecular anions calculated with the chemical model (multiplied by 0.0003, 5, 15, 0.03, and 0.05 for CN, C2H-, C4H-, C6H-, and C5N-, respectively). The abundances are expressed as number of molecules per cubic centimeter. The angular distance is given in the top axis for an assumed distance to IRC +10216 of 120 pc. |
|
| Open with DEXTER | |
To obtain a more reliable estimate of the abundance and excitation
conditions of CN- in IRC +10216, we
carried out radiative
transfer calculations based on the LVG formalism. The physical
parameters of the envelope were taken from Agúndez
(2009). We
included the first 20 rotational levels of CN-.
The rate
coefficients for de-excitation by collisions with para-H2
were
explicitly computed by means of quantum mechanical calculations
for temperatures between 5 and 70 K and transitions involving
the
first 9 rotational levels of CN-. The
calculations are
described in Appendix A.
For collisions
with He, the rate coefficients computed for para-H2
were
scaled down by a factor of 1.37 (the ratio of the square roots of
the reduced mass of each couple of collision partners). For
transitions involving rotational levels higher than J
= 8, the
Infinite Order Sudden approximation was used. As noted above,
CN- is confined to the outer envelope of
IRC +10216. We find
that to reproduce the line profiles and relative intensities
observed, the abundance of CN- relative to H2
must peak at a
radius between 13'' and 17'' from the star. The adopted radial
distribution, with a maximum abundance relative to H2
of ![]() reached at a radius of 15'' (12'' if expressed
as a particle density, see grey thick line in
Fig. 2),
produces line profiles in reasonable
agreement with the observed ones (see
Fig. 3).
We note that since the
density decreases as the radius increases, the maximum in the
particle density is reached at smaller radii than the maximum in
the abundance relative to H2. The total column
density across
the envelope (twice the radial value) is
reached at a radius of 15'' (12'' if expressed
as a particle density, see grey thick line in
Fig. 2),
produces line profiles in reasonable
agreement with the observed ones (see
Fig. 3).
We note that since the
density decreases as the radius increases, the maximum in the
particle density is reached at smaller radii than the maximum in
the abundance relative to H2. The total column
density across
the envelope (twice the radial value) is
![]() cm-2,
in good agreement with the value derived from the
rotational diagram. In the region where most of CN-
is present
(at a radius of
cm-2,
in good agreement with the value derived from the
rotational diagram. In the region where most of CN-
is present
(at a radius of ![]()
![]() cm, where the gas
kinetic temperature is
cm, where the gas
kinetic temperature is ![]() 40 K
and the density of H2molecules is around
40 K
and the density of H2molecules is around
![]() cm-3),
the rotational
levels involved in the CN- observed transitions
are
subthermally excited. Therefore, the collision rate coefficients
utilized are found to be essential to correctly estimate the
CN- abundance in the outer layers of
IRC +10216's envelope.
cm-3),
the rotational
levels involved in the CN- observed transitions
are
subthermally excited. Therefore, the collision rate coefficients
utilized are found to be essential to correctly estimate the
CN- abundance in the outer layers of
IRC +10216's envelope.
| Figure 3:
Line profiles calculated with the LVG model (thick grey
lines) using the compact CN- abundance
distribution (thick grey
line in Fig. 2)
are compared with the observed
CN- lines (black histograms). Fits to the C6H
and SiC2 |
|
| Open with DEXTER | |
To gain some insight into the formation of CN-
in the external
layers of the molecular envelope of IRC +10216, we performed
chemical modeling calculations similar to those described by
Cernicharo et al. (2008).
The physical parameters of the envelope were taken
from Agúndez (2009). The rate
constants and branching ratios of the
reactions of anions with H, O, and N atoms, studied in the
laboratory by Eichelberger
et al. (2007), were updated according to the values
used by Cordiner & Millar (2009)
and
Walsh et al. (2009)![]() .
Photodetachment rates of molecular anions were assumed by
Millar et al. (2007)
to depend on the electron affinity of the neutral
counterpart. For CN-, we assumed the same rate
expression
adopted for C6H-, because
the neutral counterparts of both
molecules have similar electron affinities (3.862
and 3.809 eV,
respectively; Rienstra-Kiracofe
et al. 2002). Plotted in
Fig. 2
is the calculated radial distribution of
the abundances of CN- (black thin line) and some
other
molecular anions. CN- is predicted to form at a
much greater
radius than C4H-, C6H-,
C3N-, and C5N-,
because, unlike the other anions, it is not formed directly from
the radical CN but by means of the reactions of the anions Cn-(
n = 5-10) with N atoms (see also Cordiner & Millar 2009).
Since CN
is a small molecule, the rate constant for the reaction of
radiative electron attachment is likely to be very small. Here we
assumed a value of
.
Photodetachment rates of molecular anions were assumed by
Millar et al. (2007)
to depend on the electron affinity of the neutral
counterpart. For CN-, we assumed the same rate
expression
adopted for C6H-, because
the neutral counterparts of both
molecules have similar electron affinities (3.862
and 3.809 eV,
respectively; Rienstra-Kiracofe
et al. 2002). Plotted in
Fig. 2
is the calculated radial distribution of
the abundances of CN- (black thin line) and some
other
molecular anions. CN- is predicted to form at a
much greater
radius than C4H-, C6H-,
C3N-, and C5N-,
because, unlike the other anions, it is not formed directly from
the radical CN but by means of the reactions of the anions Cn-(
n = 5-10) with N atoms (see also Cordiner & Millar 2009).
Since CN
is a small molecule, the rate constant for the reaction of
radiative electron attachment is likely to be very small. Here we
assumed a value of ![]() cm3 s-1
at 300 K,
similar to that computed for C2H by Herbst & Osamura (2008).
This process
results in a too low formation rate for CN-,
more than 5 orders
of magnitude lower than that provided by the reactions of Cn-and
N atoms. The reaction of HCN and H- is also a
source of
CN- in the inner regions of the envelope, but
has only a minor
contribution (less than 0.2%) to the total amount of CN-formed
in the envelope. The anion C2H-,
on the other hand,
is solely formed by the reaction of C2H2
and H-, which
takes place in the inner regions. According to our chemical model,
CN- reaches a maximum abundance relative to H2
of
cm3 s-1
at 300 K,
similar to that computed for C2H by Herbst & Osamura (2008).
This process
results in a too low formation rate for CN-,
more than 5 orders
of magnitude lower than that provided by the reactions of Cn-and
N atoms. The reaction of HCN and H- is also a
source of
CN- in the inner regions of the envelope, but
has only a minor
contribution (less than 0.2%) to the total amount of CN-formed
in the envelope. The anion C2H-,
on the other hand,
is solely formed by the reaction of C2H2
and H-, which
takes place in the inner regions. According to our chemical model,
CN- reaches a maximum abundance relative to H2
of ![]() at a radius of
at a radius of
![]() cm, and a
total column density across the envelope of
cm, and a
total column density across the envelope of
![]() cm-2.
For C2H-, the model
predicts a fairly low column
density of
cm-2.
For C2H-, the model
predicts a fairly low column
density of ![]() cm-2,
distributed within the
innermost 1016 cm. These results agree
with the recent
chemical model of Cordiner &
Millar (2009), who predicted that both CN-and
C2H- could be detected in
the circumstellar envelope of
IRC +10216.
cm-2,
distributed within the
innermost 1016 cm. These results agree
with the recent
chemical model of Cordiner &
Millar (2009), who predicted that both CN-and
C2H- could be detected in
the circumstellar envelope of
IRC +10216.
The abundance and column density predicted for CN- by the chemical model is in reasonable agreement with the value derived from the observed lines and the LVG model. However, the calculated spatial distribution differs markedly from that derived by the observations (see Fig. 2). By adopting the CN- abundance distribution obtained with the chemical model, the resulting line profiles exhibit important discrepancies from the observed ones. While the calculated absolute line intensities are about the same order of magnitude as those observed, significant disagreements between the relative intensities and the line profiles are found. The calculated line intensity decreases too rapidly when going from the J = 1-0 to the J = 3-2 line, and the computed line profiles are much too U-shaped, with nearly all the emission predicted to occur at the line edges (i.e. at the terminal expansion velocity). These discrepancies arise because the chemical model predicts that CN- is present in a region of the circumstellar envelope that is too far from the central star. An abundance distribution more compact than predicted by our chemical model may arise if the envelope is not modeled as being smooth, but as having density-enhanced shells. Cordiner & Millar (2009) recently studied the effect of these density enhancements on the radial distribution of molecular abundances and found that molecules formed in the outer envelope would concentrate at the position of the first and/or second shells, located at 15 and 27'', respectively.
The chemical model predicts C2H-
to be distributed over an 8'' diameter region (see
Fig. 2)
with a total
column density of ![]() cm-2.
Once averaged
over the 14.6'' beam of the IRAM 30-m telescope at the
frequency
of the J = 2-1 transition, the calculated
column density is
about 3 times lower than, and thus consistent with, the 3
cm-2.
Once averaged
over the 14.6'' beam of the IRAM 30-m telescope at the
frequency
of the J = 2-1 transition, the calculated
column density is
about 3 times lower than, and thus consistent with, the 3![]() upper limit
derived from the non-detection of the J =
2-1 line.
upper limit
derived from the non-detection of the J =
2-1 line.
The identification of CN- in IRC +10216 with a relatively large anion-to-neutral abundance ratio (0.25%) suggests that it may be detectable in other astronomical sources. Upper limitsto the CN-/CN abundance ratio as low as 0.2-2% were obtained in TMC-1, L1527, Barnard 1, and the Orion Bar in a previous search for the J = 2-1 transition by Agúndez et al. (2008). More sensitive observations would be needed if the abundance of CN- in other sources is similar to that found in IRC +10216.
The high abundance of CN- compared to that of C2H-demonstrates the efficiency of the reactions of N atoms and large carbon anions. A more sensitive search for C2H- might support this alternative scheme for the formation of anions in space, and perhaps explain the low observed abundance of C4H- relative to C3N-.
AcknowledgementsWe acknowledge R. Chamberlin and T. G. Phillips for their kind help during a previous search of the CN- J = 3-2 transition with the Caltech Submillimeter Observatory (CSO). We are also grateful to the astronomers that helped with the observations during the 2009 winter HERA pool at the IRAM 30-m telescope, among them F. S. Tabatabaei, E. De Beck, G. Bañó, and J. Rodón. M.A. is supported by a Marie Curie Intra-European Individual Fellowship within the European Community 7th Framework Programme under grant agreement n
235753. J.R.G. is supported by a Ramón y Cajal research contract from the Spanish MICINN and co-financed by the European Social Fund. J.K. acknowledges the partial financial supports from the University Complutense of Madrid/Grupo Santander under the program of Movilidad de Investigadores Extranjeros and from the U.S. National Science Foundation under Grant No. CHE-0848110 to M. H. Alexander. This project has been partly financed by the Spanish MICINN grants Consolider-Ingenio 2010 CSD2009-00038, AYA2009-07304, and CTQ2008-02578-BQU.
References
- Agúndez, M. 2009, Ph.D. Thesis, Universidad Autónoma de Madrid [Google Scholar]
- Agúndez, M., Cernicharo, J., Guélin, M., et al. 2008, A&A, 478, L19 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Amano, T. 2008, J. Chem. Phys., 129, 244305 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Arthurs, A. M., & Dalgarno, A. 1960, Proc. R. Soc. London, Ser. A, 256, 540 [Google Scholar]
- Botschwina, P., Seeger, S., Mladenovic, M., et al. 1995, Int. Rev. Phys. Chem., 14, 169 [CrossRef] [Google Scholar]
- Boys, S. F., & Bernardi, F. 1970, Mol. Phys., 19, 553 [NASA ADS] [CrossRef] [Google Scholar]
- Brünken, S., Gupta, H., Gottlieb, C. A., et al. 2007a, ApJ, 664, L43 [NASA ADS] [CrossRef] [Google Scholar]
- Brünken, S., Gottlieb, C. A., Gupta, H., et al. 2007b, A&A, 464, L33 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Guélin, M., Agúndez, M., et al. 2007, A&A, 467, L37 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Guélin, M., Agúndez, M., et al. 2008, ApJ, 688, L83 [NASA ADS] [CrossRef] [Google Scholar]
- Cordiner, M. A., & Millar, T. J. 2009, ApJ, 697, 68 [NASA ADS] [CrossRef] [Google Scholar]
- Eichelberger, B., Snow, T. P., Barckholtz, C., & Bierbaum, V. M. 2007, ApJ, 667, 1283 [NASA ADS] [CrossRef] [Google Scholar]
- Gottlieb, C. A., Brünken, S., McCarthy, M. C., & Thaddeus, P. 2007, J. Chem. Phys., 126, 191101 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Herbst, E., & Osamura, Y. 2008, ApJ, 679, 1670 [Google Scholar]
- Hutson, J. M., & Green, S. 1994, MOLSCAT computer code, version 14, Collaborative Computational Project No. 6 of the Science and Engineering Research Council (UK) [Google Scholar]
- Izuha, M., Yamamoto, S., & Saito, S. 1994, Spectrochim. Acta A, 50, 1371 [NASA ADS] [CrossRef] [Google Scholar]
- Knowles, P. J., Hampel, C., & Werner, H.-J. 1993, J. Chem. Phys., 99, 5219 [NASA ADS] [CrossRef] [Google Scholar]
- Knowles, P. J., Hampel, C., & Werner, H.-J. 2000, J. Chem. Phys., 112, 3106 [NASA ADS] [CrossRef] [Google Scholar]
- Lique, F., Tobo▯a, R., K▯os, J., et al. 2008, A&A, 478, 567 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- McCarthy, M. C., Gottlieb, C. A., Gupta, H., & Thaddeus, P. 2006, ApJ, 652, L141 [NASA ADS] [CrossRef] [Google Scholar]
- Millar, T. J., Walsh, C., Cordiner, M. A., et al. 2007, ApJ, 662, L87 [NASA ADS] [CrossRef] [Google Scholar]
- Müller, H. S. P., Schlöder, F., Stutzki, J., & Winnewisser, G. 2005, J. Mol. Struct., 742, 215 [NASA ADS] [CrossRef] [Google Scholar]
- Pickett, H. M., Poynter, R. L., Cohen, E. A., et al. 1998, J. Quant. Spec. Radiat. Transf., 60, 883 [Google Scholar]
- Remijan, A. J., Hollis, J. M., Lovas, F. J., et al. 2007, ApJ, 664, L47 [NASA ADS] [CrossRef] [Google Scholar]
- Rienstra-Kiracofe, J. C., Tschumper, G. S., & Schaefer III, H. F. 2002, Chem. Rev., 102, 231 [CrossRef] [PubMed] [Google Scholar]
- Thaddeus, P., Gottlieb, C. A., Gupta, H., et al. 2008, ApJ, 677, 1132 [NASA ADS] [CrossRef] [Google Scholar]
- Walsh, C., Harada, N., Herbst, E., & Millar, T. J. 2009, ApJ, 700, 752 [NASA ADS] [CrossRef] [Google Scholar]
- Williams, H. L., Szalewicz, K., Moszynski, R., & Jeziorski, B. 1995, J. Chem. Phys., 103, 4586 [NASA ADS] [CrossRef] [Google Scholar]
- Woon, D. E. & Dunning, T. H. 1994, J. Chem. Phys., 100, 2975 [NASA ADS] [CrossRef] [Google Scholar]
Online Material
Appendix A: CN--H2 collision rate coefficients
The potential energy surface (PES) of the CN--H2
complex
was calculated ab initio using single and double-excitation
coupled cluster method with non-iterative triple excitations
[CCSD(T)] (Knowles
et al. 2000,1993) implemented in MOLPRO![]() .
The geometry
of the system was described in the body-fixed frame and
characterized by three angles (
.
The geometry
of the system was described in the body-fixed frame and
characterized by three angles (![]() ,
,
![]() ,
,
![]() )
and
the distance R between the centers of mass of H2
and
CN-. The H2 bond distance
was fixed at r0=1.44876a0
and the CN- bond distance was varied for the
purpose of
averaging the PES over the lowest vibrational state of the CN-diatom.
The basis-set superposition error-correction counterpoise
procedure of Boys
& Bernardi (1970) was applied. The four atoms were
described by the correlation-consistent triple zeta basis-set
(aug-cc-pVTZ) of Woon
& Dunning (1994) augmented by the (3s, 2p, 1d)
midbond functions defined by Williams
et al. (1995), placed at mid-distance
between the CN- and H2
centers of mass. The final
)
and
the distance R between the centers of mass of H2
and
CN-. The H2 bond distance
was fixed at r0=1.44876a0
and the CN- bond distance was varied for the
purpose of
averaging the PES over the lowest vibrational state of the CN-diatom.
The basis-set superposition error-correction counterpoise
procedure of Boys
& Bernardi (1970) was applied. The four atoms were
described by the correlation-consistent triple zeta basis-set
(aug-cc-pVTZ) of Woon
& Dunning (1994) augmented by the (3s, 2p, 1d)
midbond functions defined by Williams
et al. (1995), placed at mid-distance
between the CN- and H2
centers of mass. The final
![]() PES
is five-dimensional, although in
this work we included only three perpendicular orientations of the
H2 molecule [
PES
is five-dimensional, although in
this work we included only three perpendicular orientations of the
H2 molecule [
![]() pairs: (0,0), (0,90),
(90,90)] to average over H2 rotations. In
addition, the PES
was averaged over the CN- internuclear distance
corresponding
to the CN- vibrational ground state wave
function. The 2-D PES
was finally obtained as an arithmetic average of three H2orientations.
The full five-dimensional PES and four-dimensional
scattering calculations will be presented elsewhere.
pairs: (0,0), (0,90),
(90,90)] to average over H2 rotations. In
addition, the PES
was averaged over the CN- internuclear distance
corresponding
to the CN- vibrational ground state wave
function. The 2-D PES
was finally obtained as an arithmetic average of three H2orientations.
The full five-dimensional PES and four-dimensional
scattering calculations will be presented elsewhere.
We considered collisions of CN- with
para-H
2(j2=0)
at
low temperatures. The rotational levels of CN-
and H2 are
designated by j1 and j2,
respectively. We used the fully
quantum close-coupling approach of Arthurs
& Dalgarno (1960). The standard
time-independent coupled scattering equations were solved using
the MOLSCAT code (Hutson
& Green 1994). Calculations were carried out at
values of the total energy ranging from 3.6 to 500 cm-1.
The
integration parameters were chosen to ensure convergence of the
cross-sections over this range. At the highest total energy
considered (500 cm-1), the CN-
rotational basis
included channels up to j1=21
to ensure convergence of the
excitation functions
![]() for transitions including up to the j1=8
rotational
level of CN-. The rotational basis of H2
was restricted
to j2=0 levels. The coupling
with the j2=2 (and higher)
states of H2 was not taken into account. As
shown by
Lique et al.
(2008), this approach is expected to yield reliable results
for the energy range considered here. From the above described
excitation functions, one can obtain the corresponding
state-resolved thermal rate coefficients by Boltzmann averaging
for transitions including up to the j1=8
rotational
level of CN-. The rotational basis of H2
was restricted
to j2=0 levels. The coupling
with the j2=2 (and higher)
states of H2 was not taken into account. As
shown by
Lique et al.
(2008), this approach is expected to yield reliable results
for the energy range considered here. From the above described
excitation functions, one can obtain the corresponding
state-resolved thermal rate coefficients by Boltzmann averaging
where k is the Boltzmann constant. To obtain precise values of the rate constants, the energy grid was chosen to be sufficiently fine to include the numerous scattering resonances. The total energy range considered in this work allows us to determine rate coefficients up to 70 K. The temperature dependence of the rate coefficients for selected de-excitation transitions is illustrated in Fig. A.1, with the values given in Table A.1.
![\begin{figure}
\par\includegraphics[angle=0,scale=0.475]{15186fig4.eps}
\end{figure}](/articles/aa/full_html/2010/09/aa15186-10/Timg45.png)
|
Figure A.1: Collisional de-excitation rate coefficients of CN- by para-H2 are shown as a function of temperature for the J = 1-0, 2-1, 2-0, and 3-1 rotational transitions of CN-. |
| Open with DEXTER | |
Table A.1: CN--H2 collision rate coefficients (10-10cm3 s-1).
Footnotes
- ... anion
![[*]](/icons/foot_motif.png)
- Based on observations carried out with the IRAM 30-m telescope. IRAM is supported by INSU/CNRS (France), MPG (Germany), and IGN (Spain).
- ...
![[*]](/icons/foot_motif.png)
- Appendix is only available in electronic form at http://www.aanda.org
- ...Walsh et al. (2009)
![[*]](/icons/foot_motif.png)
- http://www.physics.ohio-state.edu/ eric/research.html
- ... MOLPRO
![[*]](/icons/foot_motif.png)
- MOLPRO, version 2006.1, a package of ab initio programs, H.-J. Werner, P. J. Knowles, R. Lindh, F. R. Manby, M. Schütz, P. Celani, T. Korona, G. Rauhut, R. D. Amos, A. Bernhardsson, A. Berning, D. L. Cooper, M. J. O. Deegan, A. J. Dobbyn, F. Eckert, C. Hampel and G. Hetzer, A. W. Lloyd, S. J. McNicholas, W. Meyer and M. E. Mura, A. Nicklass, P. Palmieri, R. Pitzer, U. Schumann, H. Stoll, A. J. Stone, R. Tarroni and T. Thorsteinsson, see http://www.molpro.net
All Tables
Table 1: Observed line parameters of CN-.
Table A.1: CN--H2 collision rate coefficients (10-10cm3 s-1).
All Figures
![\begin{figure}
\par\includegraphics[angle=0,width=7.5cm,clip]{15186fig1.eps}
\end{figure}](/articles/aa/full_html/2010/09/aa15186-10/Timg5.png)
|
Figure 1:
Spectra of IRC +10216 covering the J = 1-0
to J = 3-2transitions of CN-.
Grey horizontal boxes mark their expected
positions based on the laboratory frequencies and a linewidth of
29 km s-1. Shaded areas show
the fits to the line profiles
obtained with the CLASS method shell.
The high
spectral resolution spectrum of the J =
2-1 line shows the
expected position of the different hyperfine components with their
relative intrinsic strengths. The intensity scale is expressed as
|
| Open with DEXTER | |
| In the text | |
| |
Figure 2: Abundance distribution derived for CN- in the envelope of IRC +10216 (thick grey line labeled as ``CN- fit''), as it reproduces the CN- observed line profiles (see Fig. 3). Also shown are the abundances of CN-, CN, and other molecular anions calculated with the chemical model (multiplied by 0.0003, 5, 15, 0.03, and 0.05 for CN, C2H-, C4H-, C6H-, and C5N-, respectively). The abundances are expressed as number of molecules per cubic centimeter. The angular distance is given in the top axis for an assumed distance to IRC +10216 of 120 pc. |
| Open with DEXTER | |
| In the text | |
| |
Figure 3:
Line profiles calculated with the LVG model (thick grey
lines) using the compact CN- abundance
distribution (thick grey
line in Fig. 2)
are compared with the observed
CN- lines (black histograms). Fits to the C6H
and SiC2 |
| Open with DEXTER | |
| In the text | |
![\begin{figure}
\par\includegraphics[angle=0,scale=0.475]{15186fig4.eps}
\end{figure}](/articles/aa/full_html/2010/09/aa15186-10/Timg45.png)
|
Figure A.1: Collisional de-excitation rate coefficients of CN- by para-H2 are shown as a function of temperature for the J = 1-0, 2-1, 2-0, and 3-1 rotational transitions of CN-. |
| Open with DEXTER | |
| In the text | |
Copyright ESO 2010
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.