| Issue |
A&A
Volume 506, Number 2, November I 2009
|
|
|---|---|---|
| Page(s) | 601 - 608 | |
| Section | Astrophysical processes | |
| DOI | https://doi.org/10.1051/0004-6361/200912850 | |
| Published online | 27 August 2009 | |
A&A 506, 601-608 (2009)
Methylammonium methylcarbamate thermal formation in interstellar ice analogs: a glycine salt precursor in protostellar environments
J.-B. Bossa1 - F. Duvernay1 - P. Theulé1 - F. Borget1 - L. d'Hendecourt2 - T. Chiavassa1
1 - Laboratoire de Physique des
Interactions Ioniques et Moléculaires (PIIM), Université de Provence et CNRS, UMR6633, Centre de
Saint-Jérôme, Case 252, Avenue Escadrille Normandie-Niémen, 13397
Marseille, France
2 -
Institut d'Astrophysique Spatiale (IAS), UMR8617, CNRS, B�t 121, Université Paris-Sud, 91405 Orsay Cedex, France
Received 8 July 2009 / Accepted 22 July 2009
Abstract
Context. Analyses of dust cometary grains collected by the Stardust spacecraft have shown the presence of amines and amino acids molecules, and among them glycine (NH2CH2COOH). We show how the glycine molecule could be produced in the protostellar environments before its introduction into comets.
Aims. We study the evolution of the interstellar ice analogues affected by both thermal heating and vacuum ultraviolet (VUV) photons, in addition to the nature of the formed molecules and the confrontation of our experimental results with astronomical observations.
Methods. Infrared spectroscopy and mass spectrometry are used to monitor the evolution of the H2O:CO2:CH3NH2 and CO2:CH3NH2 ice mixtures during both warming processes and VUV photolysis.
Results. We first show how carbon dioxide (CO2) and methylamine (CH3NH2) thermally react in water-dominated ice to form methylammonium methylcarbamate [CH3NH3+][CH3NHCOO-] noted C. We then determine the reaction rate and activation energy. We show that C thermal formation can occurs in the 50-70 K temperature range of a protostellar environment. Secondly, we report that a VUV photolysis of a pure C sample produces a glycine salt, methylammonium glycinate [CH3NH3+][NH2CH2COO-] noted G. We propose a scenario explaining how C and subsequently G can be synthesized in interstellar ices and precometary grains.
Conclusions. [CH3NH3+][CH3NHCOO-] could be readily formed and would act as a glycine salt precursor in protostellar environments dominated by thermal and UV processing. We propose a new pathway leading to a glycine salt, which is consistent with the detection of glycine and methylamine within the returned samples of comet 81P/Wild 2 from the Stardust mission.
Key words: astrochemistry - ISM: molecules - methods: laboratory
1 Introduction
Icy grains in the ISM play an important role in the chemistry of dense molecular clouds. They provide a surface upon which atoms and molecules can freeze out, forming icy mantles over the underlying silicates (O-rich) or carbonaceous (C-rich) grains. The current inventory of molecules within the icy grain mantles as inferred by ISO (Infrared Space Observatory), includes water (H2O), the dominant species, carbon monoxide (CO), carbon dioxide (CO2), methanol (CH3OH), ammonia (NH3), and traces of other species (Dartois 2005).These icy grains evolve from dense molecular clouds to planetary systems. During a molecular cloud collapse, a protostar surrounded by a gas and dust envelope is formed. This induces a warming up of icy grains and the reactions between frozen molecules lead to refractory compounds that remain on dust grains, while the most volatile compounds are delivered into the gas phase (hot corino, where the temperatures reach 100-200 K) (Cecarelli 2008). With time, the gas and dust envelope dissipates forming a protoplanetary disk, where the dust grains are distributed along the mid-plane. By means of yet unknown mechanisms, dust grains coagulate to form larger particles that eventually become planets, comets, and meteorites. During this evolution, organic material within grains experiences different types of chemical alteration (thermal processes, cosmic rays, and ultraviolet irradiations) depending on their location within star-forming regions.
Here, we are interested in the formation of the simplest amino acid, glycine (NH2CH2COOH), in protostellar environments. Numerous tentative detections of interstellar glycine have been reported but its identification has not yet been confirmed (Kuan et al. 2003; Snyder et al. 2005). Meteoritic glycine has been identified in both CM- and CI-type carbonaceous chondrites such as Murchison, Murray, Orgeuil, and Ivuna by using highly sensitive analytical techniques (Kvenvolden et al. 1970; Cronin & Pizzarello 1997; Botta & Bada 2002; Ehrenfreund et al. 2001a; Lawless 1972). In cometary coma, approximately 25 molecules have been detected by radio astronomy (Despois 1999; Bockelée-Morvan et al. 2000; Crovisier et al. 1998; Irvine et al. 2000), but not glycine (Crovisier et al. 2004; Ehrenfreund et al. 2002). Finally, within the returned samples of comet 81P/Wild 2 from the Stardust mission, traces of glycine and ![]() -alanine have been detected as well as amines such as methylamine and ethylamine (Sandford et al. 2006; Glavin 2008). An open question is whether glycine can be synthesized in these environments.
-alanine have been detected as well as amines such as methylamine and ethylamine (Sandford et al. 2006; Glavin 2008). An open question is whether glycine can be synthesized in these environments.
Glycine can be formed in the laboratory by Strecker synthesis involving hydrogen cyanide (HCN), ammonia (NH3), and formaldehyde (H2CO). The Bucherer-Bergs synthesis is close to that of the Strecker synthesis with additional carbon dioxide (CO2). These reactions can occur in laboratory VUV photolysis and thermal processes experiments performed on interstellar ice analogs (Munoz Caro et al. 2002; Bernstein et al. 2002; Elsila et al. 2007). After warming the irradiated ice to room temperature and hydrolyzing the residu obtained, glycine and other amino acids have been identified after GC-MS analysis. Other fruitful attempts to form interstellar glycine have been performed in the laboratory involving either ammonia (NH3) and acetic acid (CH3COOH) or methylamine (CH3NH2) and carbon dioxide (CO2) mixtures, by electron bombardments at 10 K (Lafosse 2006; Holtom et al. 2005). Finally, the VUV irradiation of methylamine (CH3NH2) and carbon dioxide (CO2) adsorbed onto a water ice surface at 56 K has also been studied, using the analysis techniques of reactive ion scattering and low energy sputtering and resulting in the identification of the amino acid glycine or the relative isomers (Lee et al. 2009). No mechanism has yet been proposed and the authors do not take into account the thermal activity of their system at 56 K.
Methylamine is an astrophysical important molecule, which has been detected by millimeter wave observations of the Sagittarius B2 and Orion A molecular clouds (Kaifu et al. 1974; Fourikis et al. 1974). Methylamine can be produced inside icy grains from the vacuum ultraviolet photolysis of other relatively abundant molecules, i.e., methane (CH4) and ammonia (NH3) (Garder & McNesby 1980; Ogura et al. 1988), or methylium ion (CH3+) and ammonia (NH3) (Herbst 1985), or from a hydrogenation series based on the cyanide radical (CN), hydrogen cyanide (HCN), and methanimine (H2C = NH) (Godfrey et al. 1973). One can also imagine that gas phase methylamine can simply stick onto icy grains. Methylamine is understood to be present on icy grains at concentrations of less than 1% relative to water. This is too low for its detections by remote infrared observations (Holtom et al. 2005).
In this paper, we provide experimental evidence of a glycine salt precursor formation from methylamine (CH3NH2) and carbon dioxide (CO2). A thermal reaction between both molecules in a water-dominated ice forms methylammonium methylcarbamate [CH3NH3+][CH3NHCOO-]. The photochemical behaviour of methylammonium methylcarbamate is studied independently. The optimal analysis conditions are achieved by the VUV irradiation at 10 K of a pure sample of methylammonium methylcarbamate (i.e., without water). We therefore show that by means of VUV irradiation, methylammonium methylcarbamate [CH3NH3+][CH3NHCOO-] is converted into a glycinate salt, methylammonium glycinate [CH3NH3+][NH2CH2COO-]. We propose a plausible scenario to explain the formation of glycine in protostellar environments and its possible introduction in comets or meteorites.
2 Description of experimental methods
The gases that we use include carbon dioxide (CO2) (Linde, purity 99.9995%), methylamine (CH3NH2) (Air Liquide, purity 99.9995%), and water that is distilled and purified by several freeze-thaw cycles within a primary vacuum. Gas mixtures are prepared in a single primary vacuum-pumped glass mixing ramp (10-3 mbar) at room temperature. The relative molecular abundances are obtained by standard manometric techniques using partial vapour pressure and from infrared spectra by estimating the column density of each component of the ices. These mixtures are introduced into a high vacuum chamber (10-7 mbar) pumped by a turbomolecular pump, containing a rotating gold-plated metal surface cooled to 10 K by a model 21 CTI Cryogenics cold head. We use the following relationA H2O:CO2:CH3NH2 = 10:3:0.5 ice analog is deposited and then heated to 4 K min-1 to simulate the thermal effects of a star-forming region. The sample chemical evolution induced by thermal and photochemical processes is monitored by infrared spectroscopy and mass spectrometry. To determine the rate constant and activation energy of the thermal reaction, kinetic studies are performed at fixed temperature by means of the isolation method. For each isothermal experiment, the initial gas mixture is condensed at 10 K, where the rate constant is too low to observe any reactivity between reactants. The warming of the ice to the desired temperature is then achieved rapidely to minimize the thermal effects between the temperature of deposition and the final temperature reached.
Infrared spectra are acquired by a FTIR spectrometer (NICOLET Magna 750) and recorded in the reflection mode in the 4000-650 cm-1 range. A typical spectrum has 1 cm-1 resolution and is averaged over 100 interferograms. A RGA quadrupole mass spectrometer (MKS Microvision-IP plus) is used to obtain information about the molecules delivered in the gaseous phase. The mass spectra are recorded between 1 and 80 amu (atomic mass units). The ionization source is defined to have a 70 eV electronic impact energy.
Ultraviolet photolysis experiments are performed using a microwave-discharge hydrogen flow lamp (Opthos instruments). The UV photon flux reaching the sample is
![]() photons cm-2 s-1. One hour of UV irradiation in the laboratory simulates the processing of interstellar ices for
photons cm-2 s-1. One hour of UV irradiation in the laboratory simulates the processing of interstellar ices for
![]() years in a dense cloud (UV flux about
years in a dense cloud (UV flux about
![]() photons cm-2 s-1) (Prasad & Tarafdar 1983; Gredel et al. 1989). The lamp is mounted directly onto the sample chamber and the UV photons are transmitted through a MgF2 window (transparent at the radiation range
photons cm-2 s-1) (Prasad & Tarafdar 1983; Gredel et al. 1989). The lamp is mounted directly onto the sample chamber and the UV photons are transmitted through a MgF2 window (transparent at the radiation range
![]() nm).
nm).
Pure methylammonium methylcarbamate used for the characterization and ultraviolet photolysis is thermally obtained from a CO2:CH3NH2 = 1:5 binary ice mixture deposited at 10 K (Bossa et al. 2008a). To avoid the photoproducts of the initial mixture, the sample is purified by warming and after the sublimation of the remaining carbon dioxide and methylamine at 200 K, pure methylammonium methylcarbamate is retained on the deposition surface. The sample is finally cooled to 10 K before being vacuum ultraviolet photolyzed.
To characterize the photochemical products formed, we record the infrared spectra of pure methylcarbamic acid (CH3NHCOOH), following the procedure described previously (Bossa et al. 2008a) and methylammonium glycinate [CH3NH3+][NH2CH2COO-] according to the procedure described below.
Methylammonium glycinate is obtained by warming a methylamine and neutral glycine binary ice mixture. Glycine (Sigma-Aldrich, purity 99%) is warmed from a glass tube mounted directly onto the sample chamber and sublimes in its non-ionic form (NH2CH2COOH). It can then be channelled by a methylamine carrier gas. After condensing the gas mixture at 10 K, the sample is warmed to 150 K. After sublimation of the remaining methylamine, pure methylammonium glycinate is formed from an acid-base reaction type. Subsequently, the methylammonium glycinate sample is cooled to 10 K before identification. Comparisons are made with the ATR spectrum of sodium glycinate [Na+, NH2CH2COO-] (Sigma-Aldrich, purity 99%) to facilitate its characterization.
![\begin{figure}
\par\includegraphics[width=9cm,clip]{12850fg1}
\end{figure}](/articles/aa/full_html/2009/41/aa12850-09/Timg14.png) |
Figure 1: Formation of methylammonium methylcarbamate [CH3NH3+] [CH3NHCOO-], from the thermal activation of carbon dioxide (CO2) and methylamine (CH3NH2) in a water-dominated ice. a) Infrared spectra of a H2O:CO2:CH3NH 2=10:3:0.5 ice film deposited at 10 K then recorded, b) after heating to 120 K, c) before water desorption at 180 K, d) after the complete desorption of water ice at 200 K, and e) infrared spectra of pure methylammonium methylcarbamate at 10 K obtained from a thermal process of a CH3NH2:CO2 = 5:1 ratio. The C abbreviation refers to methylammonium methylcarbamate and D refers to the dimer form of methylcarbamic acid (Bossa et al. 2008a). |
| Open with DEXTER | |
Table 1: Infrared band position (cm-1) and assignment of pure methylammonium methylcarbamate (C) at 10 K, pure methylammonium glycinate (G) at 10 K, pure methylamine at 10 K and pure methylcarbamic acid.
3 Results
3.1 Formation of methylammonium methylcarbamate (C) in a water-dominated ice
Carbamates are chemical products with the general structure [R-NHCO2-]. In aqueous solution, carbon dioxide (CO2) and primary amines (R-NH2) yield alkylammonium alkylcarbamates [R-NH3+][R-NHCO2-] (DellAmico et al. 2003) according to the reaction described below:
We have shown that the simplest carbamate [NH2CO2-] could be thermally produced from icy grains containing NH3 and CO2 (Bossa et al. 2008b). From a CO2:CH3NH2 = 1:5 binary ice mixture, pure methylammonium methylcarbamate [CH3NH3+][CH3NHCOO-] is thermally produced (Bossa et al. 2008a). The infrared spectrum of C is presented in Fig. 1e, and the vibrational bands are reported in Table 1. However, a CO2:CH3NH2 = 1:5 mixture is not a realistic astrophysical ice because we expect CO2 to be in excess compared to CH3NH2. Futhermore, both molecules should be diluted in a water-dominated environment. We report (Fig. 1) the results of the thermal evolution of a H2O:CO2:CH3NH2 = 10:3:0.5 ice analog mixture deposited at 10 K, which is a more astrophysicaly relevant ice.
![\begin{figure}
\par\includegraphics[width=8.8cm,clip]{12850fg2}
\end{figure}](/articles/aa/full_html/2009/41/aa12850-09/Timg26.png) |
Figure 2: Electronic impact mass spectra (70 eV) during the methylammonium methylcarbamate [CH3NH3+][CH3NHCOO-] decomposition at 248 K. |
| Open with DEXTER | |
3.2 Determination of the rate constants (k) and the activation energy (E a) of the methylammonium methylcarbamate thermal formation in a water-dominated ice
The rate constants k for the thermal reaction described in Eq. (2) in a water-dominated ice at different temperatures are investigated in the 80-110 K temperature range:We follow the time evolution of carbon dioxide infrared absorption band at 2340 cm-1 for specific temperatures. The determination of the rate law is realized using the isolation method, where methylamine is in excess within the mixture. We observe that the integrated absorption band of carbon dioxide (
where (
The partial order relative to the molar fraction of methylamine,
Both values are useful to estimate the set of k values in the temperature range of protostellar environments:
Table 2: Reaction rate k measured for methylammonium methylcarbamate formation at a fixed temperature.
![\begin{figure}
\par\includegraphics[width=9cm,clip]{12850fg3}
\end{figure}](/articles/aa/full_html/2009/41/aa12850-09/Timg58.png) |
Figure 3:
Linear fit to the Arrhenius plot of |
| Open with DEXTER | |
The set of CO2 half-times onto interstellar icy grains are listed in Table 3. Table 3 shows the comparison between these values and the residence times of CO2 on water ice as a function of the corresponding temperature. This comparison is helpful for determining the possibility of the methylammonium methylcarbamate thermal formation on interstellar icy grains located in protostellar environments during a typical time of 106-107 years.
3.3 Photolysis of pure methylammonium methylcarbamate (C) at low temperature
The photolysis of methylammonium methylcarbamate is achieved independently of its thermal formation in water-dominated ice. Pure methylammonium methylcarbamate photolysis at 10 K gives rise to new infrared absorption bands relative to newly formed species. In Fig. 4e, we display the difference spectrum recorded between 240 min of VUV photolysis and before any photolysis, where the remaining methylammonium methylcarbamate contributions have been subtracted. Hence, Fig. 4e depicts only the infrared features relative to the photochemical products. We identify methylcarbamic acid (CH3NHCOOH) (Fig. 4a) and methylamine (CH3NH2) (Fig. 4b) by comparing the infrared spectra of the respective pure samples (Durig et al. 1968; Bossa et al. 2008a). The formation of both products is consistent with a proton transfer induced by ultraviolet photons from methylammonium cation [CH3NH3+] to methylcarbamate anion [CH3NHCOO-] as described below:The infrared spectra corresponding to both products cannot explain the difference spectrum of the irradiated sample. Some features, such as the most intense band at 1565 cm-1 are not yet assigned and suggest the formation of a third species.
Table 3: Comparison between the calculated CO2 half-times involved in the methylammonium methylcarbamate (C) thermal formation and the calculated residence times of CO2 on water ice at different temperatures.
![\begin{figure}
\par\includegraphics[width=9cm,clip]{12850fg4}
\end{figure}](/articles/aa/full_html/2009/41/aa12850-09/Timg71.png) |
Figure 4: Formation of methylammonium glycinate [CH3NH3+] [NH2CH2COO-] from the vacuum ultraviolet photolysis of pure methylammonium methylcarbamate [CH3NH3+][CH3NHCOO-] at low temperature (10 K). Comparison between the infrared spectra of a) pure methylcarbamic acid (CH3NHCOOH); b) pure methylamine (CH3NH2) at 10 K; c) pure methylammonium glycinate [CH3NH3+][NH2CH2COO-] at 10 K; d) sum of spectra a) + b) + c) and e) difference spectrum after VUV photolysis (240 min) minus before VUV photolysis with the remaining methylammonium methylcarbamate (C) contributions subtracted. The minor discrepancy between e) and d) spectra can be explained by environment effects that are not taken into account in the sum spectra. The infrared absorption feature (*) is relative to the dimer form of methylcarbamic acid (Bossa et al. 2008a) and its pure infrared spectra has been omitted for clarity. |
| Open with DEXTER | |
![\begin{figure}
\par\includegraphics[width=9cm,clip]{12850fg5}
\end{figure}](/articles/aa/full_html/2009/41/aa12850-09/Timg72.png) |
Figure 5:
Comparison between the infrared spectra of a) ATR spectrum of the glycine sodium salt [Na+, NH2CH2COO-] at room temperature; b) methylammonium glycinate [CH3NH3+][NH2CH2COO-] at 10 K; c) difference spectrum after VUV photolysis (240 min) minus before VUV photolysis with the remaining methylammonium methylcarbamate (C) contributions subtracted. Comments: Vibration mode: stretching ( |
| Open with DEXTER | |
Glycine can exist in different forms depending on the environment (acid, neutral/zwitterionic, or basic). A comparison with the spectra of glycine, in either a neutral (NH2CH2COOH) or a zwitterionic form (NH3+CH2COO-), is indecisive (Gomez-Zavaglia & Fausto 2003; Rosado et al. 1998). In a basic environment, glycine exits as a glycinate salt [NH2CH2COO-]. The infrared spectrum of commercial sodium glycinate [Na+, NH2CH2COO-] (Fig. 5a) is close to the unknown infrared features. Closer agreement is obtained with the infrared spectrum of methylammonium glycinate [CH3NH3+][NH2CH2COO-] (Figs. 4c and 5b). The infrared absorption bands of methylammonium glycinate (noted G) are listed with their assignments in Table 1. These assignments are confirmed by comparing the infrared spectrum of the sodium glycinate (Fig. 5a) with that of methylammonium glycinate (Fig. 5b). The main differences with the sodium glycinate spectrum come from the methylammonium [CH3NH3+] counter-ion, which adds several features in the infrared spectrum.
The detection of G from the pure C photolysis is then straightforward, mainly by means of the methylammonium [CH3NH3+] vibrational bands (Waldron 1953; Bossa et al. 2008a; Castellucci 1974; Cabana & Sandorfy 1962) located between 3200 and 2500 cm-1 as well as the most intense G contributions corresponding to the CO2- asymmetric and symmetric modes at 1565 cm-1 and 1403 cm-1 (Figs. 5b and c). A simple addition of the spectra corresponding to methylcarbamic acid (Fig. 4a), methylamine (Fig. 4b), and methylammonium glycinate (Fig. 4c) reproduces rather faithfully the photochemical product infrared spectrum (Figs. 4d and e). The minor discrepancy between both spectra can be explained by the environment effects that are not taken into account in the sum spectrum (Fig. 4d) and are known to affect both the band profiles and the band strengths.
3.4 Estimation of methylammonium glycinate (G) produced by pure methylammonium methylcarbamate (C) during the VUV photolysis
We quantify the methylammonium glycinate production by the VUV irradiation of pure methylammonium methylcarbamate. This is achieved by calculating the total column density of G formed and C consumed. The quantitative analysis of G formed is derived from its infrared spectrum (Fig. 4c) adjusted to achieve the best fit to the difference spectrum (Fig. 4e). The column density of G formed is measured from the optical depth located at 1403 cm-1, corresponding to the
![]() CO2- fundamental mode but its band strength is unknown. This value is determined by the thermal conversion of a pure sample of methylammonium glycinate into zwitterionic glycine (NH3+CH2COO-), noted Z, represented by
CO2- fundamental mode but its band strength is unknown. This value is determined by the thermal conversion of a pure sample of methylammonium glycinate into zwitterionic glycine (NH3+CH2COO-), noted Z, represented by
We choose a Z band at 1414 cm-1 without any overlap to determine its band strength (AZ). Preparing a calibrated KBr pellet of commercial Z, we found a AZ value of
After irradiation (240 min), 50% of the C is consumed by the photolysis. From these measurements, we can obtain a rough quantitative estimation of the photolysis yield of glycinate produced to be about 32%. Nevertheless, the same irradiation experiment with methylammonium methylcarbamate diluted in water should lower this photolysis yield.
4 Discussion
During their lifetime in a protostellar environment (106-107 years), interstellar ices can be submitted to both thermal and UV photolysis processing that change the grain chemical composition.
Our investigations show that a glycine isomer salt, methylammonium methylcarbamate (C) can be produced in a water-dominated ice from the thermal reaction between carbon dioxide and methylamine. The relative abundance of carbon dioxide on icy grains is about 20% compared to water (Ehrenfreund & Charnley 2000; Charnley et al. 2001). Methylamine is observed in the gas-phase of the interstellar medium, and is expected to efficiently condense onto the icy grain surface. Because of its non-detection in solid-state materials with current remote infrared observational techniques, the molar fraction of methylamine in icy grains should be at concentrations of less than 1% relative to water.
The calculation of Arrhenius parameters (A and Ea) and rate constants (k) relative to the thermal reaction in a water-dominated ice allow us to determine by extropolation the possibility of C formation in protostellar environments. Table 3 compares the CO2 half-times involved in the reaction with the CO2 residence times onto water ice in the corresponding temperature range (Sandford & Allamandola 1993). The CO2 half-times are fast in the 50-70 K temperature range (10-2-10-3 years) compared to the longer or equivalent CO2 residence time (104-10-3 years). The CO2 half-times are also faster than the typical ice lifetime (106-107 years). Methylammonium methylcarbamate (C) is hence likely to be present in the icy grains warmed in the 50-70 K temperature range of protostellar environments. Since cometary ices are related to interstellar ices, the same assumption should be applicable to them. In the hot core regions, temperatures readily exceed 50 K and are conclusive to C formation on the icy grains. In the inner protostellar disk, as the temperature decreases in the radial direction, the 50-70 K temperature range corresponds approximatively to 30-15 UA from the young sun (Hayashi 1981). This implies that the icy materials accreted in the 30-15 UA distance range should provide ideal conditions for C formation. Assuming that the abundance of CH3NH2 into icy grains is less than 1% relative to water and that CH3NH2 is totally consumed in the thermal reaction during the typical ices lifetime, we predict the abundance of C in icy grains to be less than 0.50% relative to water. This implies that C may not be detectable by current remote infrared observational techniques.
Methylammonium methylcarbamate is more refractory than H2O or the precursor molecules, implying that it acts as a CO2 and CH3NH2 molecules reservoir. This could partially explain the CO2 depletion in the protostellar hot core gas (Charnley et al. 2001). Methylammonium methylcarbamate remains stable for a wide range of temperatures but, for T > 240 K, it decomposes successively into methylcarbamic acid (CH3NHCOOH), then into CH3NH2 and CO2 as clearly illustrated by mass spectrometry by their respective peaks at
![]() (CH3NHCOOH
(CH3NHCOOH
![]() ),
),
![]() (CO
(CO
![]() ), and
), and
![]() (CH3NH
(CH3NH
![]() ). The instability of methylcarbamic acid (CH3NHCOOH) in the gas phase makes its detection in the interstellar medium difficult by radio astronomy.
). The instability of methylcarbamic acid (CH3NHCOOH) in the gas phase makes its detection in the interstellar medium difficult by radio astronomy.
Pure methylammonium methylcarbamate acts as a glycine salt precursor in VUV environments. We determine the yield of the isomerization process induced by ultraviolet photons to be about 32% in anhydrous conditions. This was inferred independently of the C thermal formation to facilitate the characterization of the photochemical products. This yield should drastically decline during similar VUV irradiation in a water-dominated ice. The pure C photolysis is also achieved at 10 K rather than in the 50-70 K temperature range so as not to combine both irradiation and thermal effects. Since CH3NH2 and CO2 are primary and secondary photochemical products, respectively, there would be competition between the processes of the thermal formation of C and its photolysis. This increases the difficulties in both the characterization and the quantitative approach. The 240 min irradiation time corresponds to
![]() years in a dense cloud (Prasad & Tarafdar 1983; Gredel et al. 1989). We assume that the upper relative abundance of C produced in the interstellar ices is 0.50%. During a period of
years in a dense cloud (Prasad & Tarafdar 1983; Gredel et al. 1989). We assume that the upper relative abundance of C produced in the interstellar ices is 0.50%. During a period of
![]() years, less than 32% of C is converted into G in anhydrous conditions. We therefore predict that the relative abundance of G in interstellar ices is lower than 0.16%, and it follows that its direct detection with infrared telescopes should be almost impossible.
years, less than 32% of C is converted into G in anhydrous conditions. We therefore predict that the relative abundance of G in interstellar ices is lower than 0.16%, and it follows that its direct detection with infrared telescopes should be almost impossible.
In a protostellar environment, the VUV photons emitted from the young stellar object (YSO) are probably sufficient to transform C into G, before their introduction into comets by icy grains coagulation occurring in the protostellar disk. Comets are then later able to bring prebiotic molecules (amino acids) to primitive earths (Maurette 2006; Delsemme 1994). The glycine salt formation arises inside grains. These grains can then protect G from the intense UV radiation and ensure that it survives longer. On the other hand, in the star-forming regions, the survival of gaseous phase amino acids is limited to short time (Ehrenfreund et al. 2001b). The radio-astronomical searches of gas phase glycine have therefore been unsuccessful.
Several reactional mechanisms can explain the formation of G from C during the VUV irradiation. One of the simplest is summarized in reactions of Eqs. (9), (10), and (11). The first step starts with a carbon-nitrogen bond rupture on methylcarbamate anion [CH3NHCOO-], yielding a carbon dioxide anion radical (CO2-) and a methyl amino radical (CH3NH). Then a methyl amino radical (CH3NH) rearranges itself into a more stable aminomethyl radical (CH2NH2) (Woon 2002). Finally, glycinate [NH2CH2COO-] formation is achieved by a barrierless radical-radical recombination between a carbon dioxide anion radical (CO2-) and an aminomethyl radical (CH2NH2).
A comparison can be made between our results and the analysis of cometary grains collected by the Stardust spacecraft ejected from the comet P81/Wild 2. After an acidic hydrolysis procedure, the latter analysis indeed detected traces of methylamine and glycine (Sandford et al. 2006; Glavin 2008). It was also suggested that these freely detected molecules were stored predominantly in an acid-soluble residue before any chemical treatment of the returned samples. This assumption is consistent with our observations of G, which could be one of the acid labile residues that would release free methylamine and free glycine after the chemical treatment of cometary grain samples.
A confirmation of the detection of these molecules and more specifically carbamate or glycinate, should be provided by the ROSETTA mission, including the gas analyser COSAC (COmetary SAmpling and Composition experiment) instrument onboard the lander Philae designed to identify complex organic molecules released from the comet nucleus.
5 Conclusion
Icy grains into the ISM can play an important role in prebiotic molecule formation. A glycine isomer salt [CH3NH3+][CH3NHCOO-] (C) is thermally produced from carbon dioxide (CO2) and methylamine (CH3NH2). The rate constant in a water-dominated ice is estimated to be
![]() .
This reaction can occur in a 50-70 K temperature range, which is consistent with protostellar environments. We predict that the upper limit to the abundance of C produced in the interstellar ices is 0.50% relative to water. Because of this low abundance and the gas phase decomposition, its detection is therefore impossible by current observational methods.
.
This reaction can occur in a 50-70 K temperature range, which is consistent with protostellar environments. We predict that the upper limit to the abundance of C produced in the interstellar ices is 0.50% relative to water. Because of this low abundance and the gas phase decomposition, its detection is therefore impossible by current observational methods.
The identification and the quantitative analysis of the photochemical products yielded during the pure methylammonium methylcarbamate VUV irradiation, are performed independently of its thermal formation. The presence of methylammonium glycinate [CH3NH3+][NH2CH2COO-] (G) is straightforwardly identified by an in situ analysis. Hence, in a VUV environment, [CH3NH3+][CH3NHCOO-] acts as a glycine salt precursor. We calculated the photolysis yield of G to be about 32% in anhydrous conditions. We predict that the abundance of G produced in the interstellar ices is lower than 0.16% relative to water. Its detection is also impossible with current observational methods.
We propose a new pathway leading to a glycine salt in interstellar ices. Interstellar glycine might be formed by thermal processing followed by the VUV processing of ices containing carbon dioxide and methylamine. The reactions involved in these processes are summarized below:
Our result is consistent with the detection of both glycine and methylamine within the samples returned from comet 81P/Wild 2. The pathway that we propose originates from in situ experimental evidence and does not need any specific treatment such as water extraction, acid hydrolysis, and derivatization. Methylammonium methylcarbamate or methylammonium glycinate could consist of both interstellar and cometary ices. We believe in the importance of considering thermal processing, VUV photolysis, but also cosmic-ray processing of the interstellar salts that will be produced from the primitive material. Deriving such a chemical scenario on the basis of laboratory work can help astronomers to constrain the sources in which prebiotic molecules could be detected. Acknowledgements
We thank the PCMI (Physique et Chimie du Milieu Interstellaire) program and also the CNES (Centre National d'Études Spatiales) agency for financial support. The authors would also like to thank Dr. L. Stievano as well as Pr. J. F. Lambert for useful discussions and contributions.
References
- Bernstein, M. P., Dworkin, J. P., Sandford, S. A., Cooper, G. W., & Allamandola, L. J. 2002, Nature, 416, 401 [NASA ADS] [CrossRef]
- Bockelée-Morvan, D., Lis, D. C., Wink, J., et al. 2000, A&A, 353, 1101 [NASA ADS]
- Bossa, J. B., Borget, F., Duvernay, F., Theulé, P., & Chiavassa, T. 2008a, J. Phys. Chem. A, 112, 5113 [CrossRef]
- Bossa, J. B., Theulé, P., Duvernay, F., Borget, F., & Chiavassa, T. 2008b, A&A, 492, 719 [NASA ADS] [CrossRef] [EDP Sciences]
- Botta, O., & Bada, J. L. 2002, Surveys in Geophysics, 23, 411 [NASA ADS] [CrossRef]
- Cabana, A., & Sandorfy, C. 1962, Spectrochim. Acta, 18, 843 [NASA ADS]
- Castellucci, E. 1974, J. Mol. Struct., 23, 449 [NASA ADS] [CrossRef]
- Cecarelli, C. 2008, International Astronomical Union, Organic Matter in Space, Proc. IAU Symp., 251
- Charnley, S. B., Ehrenfreund, P., & Kuan, Y.-J. 2001, Spectrochim. Acta, Part A, 57A, 685
- Cronin, J. R., & Pizzarello, S. 1997, Science, 275, 951 [NASA ADS] [CrossRef]
- Crovisier, J. 1998, Faraday Discuss., 109, 437 [CrossRef]
- Crovisier, J., Bockelée-Morvan, D., Colom, P., et al. 2004, A&A, 418, 1141 [NASA ADS] [CrossRef] [EDP Sciences]
- Dartois, E. 2005, Space Sci. Rev., 119, 293 [NASA ADS] [CrossRef]
- DellAmico, D. B., Calderazzo, F., Labella, L., Marchetti, F., & Pampaloni, G. 2003, Chem. Rev., 103, 3857 [CrossRef]
- Delsemme, A. H. 1994, Adv. Space Res., 15, 49 [CrossRef]
- Despois, D. 1999, Earth Moon Planets, 79, 103 [NASA ADS] [CrossRef]
- Durig, J. R., Bush, S. F., & Baglin, F. G. 1968, J. Chem. Phys., 49, 2106 [NASA ADS] [CrossRef]
- Ehrenfreund, P., & Charnley, S. B. 2000, ARA&A, 38, 427 [NASA ADS] [CrossRef]
- Ehrenfreund, P., Glavin, D. P., Botta, O., Cooper, G., & Bada, J. L. 2001a, PNAS, 98, 2138 [NASA ADS] [CrossRef]
- Ehrenfreund, P., Bernstein, M. P., Dworkin, J. P., Sandford, S. A., & Allamandola, L. J. 2001b, ApJ, 550, L95 [NASA ADS] [CrossRef]
- Ehrenfreund, P., Irvine, W., Becker, L., et al. 2002, Rep. Progr. Phys., 65, 1427 [NASA ADS] [CrossRef]
- Elsila, J. E., Dworkin, J. P., Bernstein, M. P., Martin, M. P., & Sandford, S. A. 2007, ApJ, 660, 911 [NASA ADS] [CrossRef]
- Fourikis, N., Tagaki, K., & Morimoto, M. 1974, ApJ, 191, 139 [NASA ADS] [CrossRef]
- Gardner, E. P., & McNesby, J. R. 1980, J. Photochem., 13, 353 [CrossRef]
- Glavin, D. P., Dworkin, J. P., & Sandford, S. A. 2008, Meteoritics & Planetary Science, 43, 399 [NASA ADS]
- Godfrey, P. D., Brown, R. D., Robinson, B. J., & Sinclair, M. W. 1973, ApL, 13, 119 [NASA ADS]
- Gomez-Zavaglia, A., & Fausto, R. 2003, Phys. Chem. Chem. Phys., 5, 3154 [CrossRef]
- Gredel, R., Lepp, S., Dalgarno, A., & Herbst, E. 1989, ApJ, 347, 289 [NASA ADS] [CrossRef]
- Hayashi, C. 1981, Prog. Theo. Phys., 70, 35 [NASA ADS] [CrossRef]
- Herbst, E. 1985, ApJ, 292, 484 [NASA ADS] [CrossRef]
- Holtom, P. D., Bennett, C. J., Osamura, Y., Mason, N. J., & Kaiser, R. I. 2005, ApJ, 626, 940 [NASA ADS] [CrossRef]
- Irvine, W. M., Schloerb, F. P., Crovisier, J., Fegley, B., & Mumma, M. J. 2000, Comets: a link between interstellar and nebular chemistry, Protostars and Planets IV, ed. V. Manning, A. Boss, & S. Russell (Tucson: University of Arizona Press)
- Kaifu, N., Morimoto, M., Nagane, K., et al. 1974, ApJ, 191, 135 [NASA ADS] [CrossRef]
- Kuan, Y. J., Charnley, S. B., Huang, H. C., Tseng, W. L., & Kisiel, Z. 2003, ApJ, 593, 848 [NASA ADS] [CrossRef]
- Kvenvolden, K., Lawless, J., Pering, K., et al. 1970, Nature, 288, 923 [NASA ADS] [CrossRef]
- Lafosse, A., Bertin, M., Domaracka, A., et al. 2006, Phys. Chem. Chem. Phys., 8, 5564 [CrossRef]
- Lawless, J., Kvenvolden, K. A., Peterson, E., Ponnamperuma, C., & Jarosewich, E. 1972, Nature, 236, 66 [NASA ADS] [CrossRef]
- Lee, C. W., Kim, J. K., Moon, E. S., Minh, Y. C., & Kang, H. 2009, ApJ, 697, 428 [NASA ADS] [CrossRef]
- Maurette, M. 2006, Micrometeorites and the mysteries of our origins, Advances in Astrobiology and Biogeophysics (Berlin, Heidelberg, New York: Springer)
- Munoz Caro, G. M., Meierhenrich, U. J., Schutte, W. A., et al. 2002, Nature, 416, 403 [NASA ADS] [CrossRef]
- Oeberg, K. I., Fraser, H. J., Boogert, A. C. A., et al. 2007, A&A, 462, 1187 [NASA ADS] [CrossRef] [EDP Sciences]
- Ogura, K., Migita, C. T., & Yamada, T. 1988, Chem. Lett., 1563
- Prasad, S., & Tarafdar, S. P. 1983, ApJ, 267, 603 [NASA ADS] [CrossRef]
- Rosado, M. T., Duarte, M. L. T. S., & Fausto, R. 1998, Vib. Spectrosc., 16, 35 [CrossRef]
- Sandford, S. A., & Allamandola, L. J. 1993, ApJ, 417, 815 [NASA ADS] [CrossRef]
- Sandford, S. A., Aleon, J., Alexander, C. M. O'D, et al. 2006, Science, 314, 1720 [NASA ADS] [CrossRef]
- Schutte, W. A., & Gerakines, P. A. 1995, Planet. Space Sci., 43, 1253 [NASA ADS] [CrossRef]
- Snyder, L. E., Lovas, F. J., Hollis, J. M., et al. 2005, ApJ, 619, 914 [NASA ADS] [CrossRef]
- Waldron, R. D. 1953, J. Chem. Phys., 21, 734 [NASA ADS] [CrossRef]
- Woon, D. E. 2002, ApJ, 571, 177 [NASA ADS] [CrossRef] (In the text)
All Tables
Table 1: Infrared band position (cm-1) and assignment of pure methylammonium methylcarbamate (C) at 10 K, pure methylammonium glycinate (G) at 10 K, pure methylamine at 10 K and pure methylcarbamic acid.
Table 2: Reaction rate k measured for methylammonium methylcarbamate formation at a fixed temperature.
Table 3: Comparison between the calculated CO2 half-times involved in the methylammonium methylcarbamate (C) thermal formation and the calculated residence times of CO2 on water ice at different temperatures.
All Figures
![\begin{figure}
\par\includegraphics[width=9cm,clip]{12850fg1}
\end{figure}](/articles/aa/full_html/2009/41/aa12850-09/Timg14.png) |
Figure 1: Formation of methylammonium methylcarbamate [CH3NH3+] [CH3NHCOO-], from the thermal activation of carbon dioxide (CO2) and methylamine (CH3NH2) in a water-dominated ice. a) Infrared spectra of a H2O:CO2:CH3NH 2=10:3:0.5 ice film deposited at 10 K then recorded, b) after heating to 120 K, c) before water desorption at 180 K, d) after the complete desorption of water ice at 200 K, and e) infrared spectra of pure methylammonium methylcarbamate at 10 K obtained from a thermal process of a CH3NH2:CO2 = 5:1 ratio. The C abbreviation refers to methylammonium methylcarbamate and D refers to the dimer form of methylcarbamic acid (Bossa et al. 2008a). |
| Open with DEXTER | |
| In the text | |
![\begin{figure}
\par\includegraphics[width=8.8cm,clip]{12850fg2}
\end{figure}](/articles/aa/full_html/2009/41/aa12850-09/Timg26.png) |
Figure 2: Electronic impact mass spectra (70 eV) during the methylammonium methylcarbamate [CH3NH3+][CH3NHCOO-] decomposition at 248 K. |
| Open with DEXTER | |
| In the text | |
![\begin{figure}
\par\includegraphics[width=9cm,clip]{12850fg3}
\end{figure}](/articles/aa/full_html/2009/41/aa12850-09/Timg58.png) |
Figure 3:
Linear fit to the Arrhenius plot of |
| Open with DEXTER | |
| In the text | |
![\begin{figure}
\par\includegraphics[width=9cm,clip]{12850fg4}
\end{figure}](/articles/aa/full_html/2009/41/aa12850-09/Timg71.png) |
Figure 4: Formation of methylammonium glycinate [CH3NH3+] [NH2CH2COO-] from the vacuum ultraviolet photolysis of pure methylammonium methylcarbamate [CH3NH3+][CH3NHCOO-] at low temperature (10 K). Comparison between the infrared spectra of a) pure methylcarbamic acid (CH3NHCOOH); b) pure methylamine (CH3NH2) at 10 K; c) pure methylammonium glycinate [CH3NH3+][NH2CH2COO-] at 10 K; d) sum of spectra a) + b) + c) and e) difference spectrum after VUV photolysis (240 min) minus before VUV photolysis with the remaining methylammonium methylcarbamate (C) contributions subtracted. The minor discrepancy between e) and d) spectra can be explained by environment effects that are not taken into account in the sum spectra. The infrared absorption feature (*) is relative to the dimer form of methylcarbamic acid (Bossa et al. 2008a) and its pure infrared spectra has been omitted for clarity. |
| Open with DEXTER | |
| In the text | |
![\begin{figure}
\par\includegraphics[width=9cm,clip]{12850fg5}
\end{figure}](/articles/aa/full_html/2009/41/aa12850-09/Timg72.png) |
Figure 5:
Comparison between the infrared spectra of a) ATR spectrum of the glycine sodium salt [Na+, NH2CH2COO-] at room temperature; b) methylammonium glycinate [CH3NH3+][NH2CH2COO-] at 10 K; c) difference spectrum after VUV photolysis (240 min) minus before VUV photolysis with the remaining methylammonium methylcarbamate (C) contributions subtracted. Comments: Vibration mode: stretching ( |
| Open with DEXTER | |
| In the text | |
Copyright ESO 2009
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.



![\begin{displaymath}
{\rm CO}_{2} + 2\ {\rm R-NH_{2}} \rightarrow {\rm [R-NH_{3}^{+}][R-NHCO_{2}^{-}]}.
\end{displaymath}](/articles/aa/full_html/2009/41/aa12850-09/img25.png)
![\begin{displaymath}
{\rm CO_{2}} + 2\ {\rm CH_{3}NH_{2}} \stackrel{k}{\longrightarrow} {\rm [CH_{3}NH_{3}^{+}][CH_{3}NHCOO^{-}]}.
\end{displaymath}](/articles/aa/full_html/2009/41/aa12850-09/img30.png)
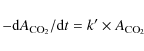
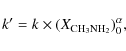
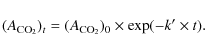
![\begin{displaymath}
{\rm [CH_{3}NH_{3}^{+}][CH_{3}NHCOO^{-}]} \stackrel{h\nu}{\longrightarrow} {\rm CH_{3}NH_{2}} + {\rm CH_{3}NHCOOH}.
\end{displaymath}](/articles/aa/full_html/2009/41/aa12850-09/img59.png)
![\begin{displaymath}
{\rm [CH_{3}NH_{3}^{+}][NH_{2}CH_{2}COO^{-}]} \stackrel{\Del...
...htarrow} {\rm CH_{3}NH_{2}} + {\rm NH_{3}^{+}CH_{2}COO^{-}}.~~
\end{displaymath}](/articles/aa/full_html/2009/41/aa12850-09/img73.png)
![\begin{displaymath}
{\rm [CH_{3}NHCOO^{-}]} + h\nu \rightarrow {\rm CH_{3}NH} + {\rm CO_{2}^{-}}
\end{displaymath}](/articles/aa/full_html/2009/41/aa12850-09/img87.png)
![\begin{displaymath}
{\rm CH_{2}NH_{2}} + {\rm CO_{2}^{-}} \rightarrow {\rm [NH_{2}CH_{2}COO^{-}]}.
\end{displaymath}](/articles/aa/full_html/2009/41/aa12850-09/img89.png)
![\begin{displaymath}
{\rm CO_{2}} + 2\ {\rm CH_{3}NH_{2}} \stackrel{\footnotesize...
...}}}{\longrightarrow} {\rm [CH_{3}NH_{3}^{+}][CH_{3}NHCOO^{-}]}
\end{displaymath}](/articles/aa/full_html/2009/41/aa12850-09/img91.png)