| Issue |
A&A
Volume 494, Number 3, February II 2009
|
|
|---|---|---|
| Page(s) | 969 - 976 | |
| Section | Interstellar and circumstellar matter | |
| DOI | https://doi.org/10.1051/0004-6361:200810863 | |
| Published online | 22 December 2008 | |
Signature of [SiPAH]+ 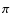 -complexes in the interstellar medium
-complexes in the interstellar medium![[*]](/icons/foot_motif.gif)
B. Joalland1,2,3,4 - A. Simon1,2 - C. J. Marsden3,4 - C. Joblin1,2
1 - Université de Toulouse, UPS, CESR, 9 avenue du colonel Roche, 31028 Toulouse Cedex 9, France
2 -
CNRS, UMR 5187, 31028 Toulouse, France
3 -
Université de Toulouse, UPS, LCPQ/IRSAMC, 118 route de Narbonne, 31062 Toulouse, France
4 -
CNRS, UMR 5626, 31062 Toulouse, France
Received 26 August 2008 / Accepted 29 October 2008
Abstract
Aims. We investigate the presence of silicon atoms adsorbed on the surface of interstellar polycyclic aromatic hydrocarbons (PAHs) to form SiPAH ![]() -complexes.
-complexes.
Methods. We used quantum chemistry calculations to obtain the structural, thermodynamic, and mid-IR properties of neutral and cationic SiPAH ![]() -complexes.
-complexes.
Results. The binding energy was found to be at least 1.5 eV for [SiPAH]+ complexes, whereas it is ![]() 0.5 eV for their neutral counterparts. From the spectral analysis of the calculated IR spectra, we found that the coordination of Si to PAH+ does not strongly affect the intensities of the PAH+ spectra, but systematically introduces blueshifts of the C-C in-plane and the C-H out-of-plane bands.
0.5 eV for their neutral counterparts. From the spectral analysis of the calculated IR spectra, we found that the coordination of Si to PAH+ does not strongly affect the intensities of the PAH+ spectra, but systematically introduces blueshifts of the C-C in-plane and the C-H out-of-plane bands.
Conclusions. The thermodynamic data calculated for [SiPAH]+ complexes show that these species are stable and can be easily formed by radiative association of Si+ and PAH species that are known to be abundant in photodissociation regions. Their mid-IR fingerprints show features induced by the coordination of Si that could account for (i) the blueshifted position of the 6.2 ![]() m AIB and (ii) the presence of satellite bands observed on the blue side of the 6.2 and 11.2
m AIB and (ii) the presence of satellite bands observed on the blue side of the 6.2 and 11.2 ![]() m AIBs. From such an assignment, we can deduce that typically 1% of the cosmic Si appears to be attached to PAHs.
m AIBs. From such an assignment, we can deduce that typically 1% of the cosmic Si appears to be attached to PAHs.
Key words: astrochemistry - ISM: abundances - ISM: molecules - ISM: lines and bands - methods: numerical
1 Introduction
Silicon is the most depleted abundant element after iron, representing a major constituent of interstellar ``standard'' dust grains (Whittet 2003), essentially in the form of silicates. Jones (2000) concluded from the analysis of depletion patterns and dust processes in different regions of the diffuse interstellar medium (ISM) that Si is more easily eroded from dust than any of the other elements. This is in line with the work of Tielens (1998), who demonstrated that a significant fraction of the elemental Si is locked up in a relatively volatile dust component with a binding energy roughly equal to 2.0 eV.
Polycyclic aromatic hydrocarbons (PAHs) that are amongst the dust components offer a large surface for atomic and molecular adsorptions. Klotz et al. (1995) have established a correlation between metal-PAH binding energies and the observed depletion of these atoms in the ISM. This model predicts that the abundance of complexed PAH molecules can explain 5-10% of the depletion of metallic atoms in the gas phase of the diffuse interstellar medium. This work also emphasizes that Fe and Si are the most abundant metals bonded on PAHs, if Si is considered as a metal although it is not in strict chemical terms.
Interstellar PAHs are revealed by their emission in the aromatic infrared bands (AIBs), which set aglow the general interstellar medium (Allamandola et al. 1985; Léger & Puget 1984). The interpretation of the AIBs has motivated a lot of observational, experimental, and theoretical studies (see Tielens 2008, for a recent review), but it is not yet entirely resolved, leaving the door open to search for new types of PAH-related species such as SiPAH ![]() -complexes.
-complexes.
Srinivas et al. (1992) and Jaeger et al. (2005) demonstrated theoretically and experimentally that the ![]() -system complex [SiC6H6]+ is more stable than all the other isomers.
Experimental studies on [SiPAH]+ complexes are scarce. In the selected-ion-flow-tube (SIFT) experiments carried out by Bohme's group, it was clearly established that the capture of ground state atomic silicon ions Si+ (2P) by benzene and naphthalene is an efficient process in the gas phase (Bohme et al. 1991). These authors also suggested a possible chemical role for the large PAH molecular surface in the catalysis of small Si-bearing molecules of astrophysical interest (Bohme et al. 1989). The radiative association of Si+ with a variety of PAHs was studied in the low-pressure conditions of a Fourier-transform-ion-cyclotron-resonance (FTICR) mass spectrometer in the group of Dunbar (Pozniac & Dunbar 1997; Dunbar et al. 1994). Charge transfer and condensation reactions (insertion of Si between C and H with loss of H) were found to compete efficiently with the association process. The results obtained in these low-pressure conditions can be a good indication of the processes that are likely to occur in the ISM; that is to say, [SiPAH]+ complexes can be formed efficiently and play a role in the chemistry there.
-system complex [SiC6H6]+ is more stable than all the other isomers.
Experimental studies on [SiPAH]+ complexes are scarce. In the selected-ion-flow-tube (SIFT) experiments carried out by Bohme's group, it was clearly established that the capture of ground state atomic silicon ions Si+ (2P) by benzene and naphthalene is an efficient process in the gas phase (Bohme et al. 1991). These authors also suggested a possible chemical role for the large PAH molecular surface in the catalysis of small Si-bearing molecules of astrophysical interest (Bohme et al. 1989). The radiative association of Si+ with a variety of PAHs was studied in the low-pressure conditions of a Fourier-transform-ion-cyclotron-resonance (FTICR) mass spectrometer in the group of Dunbar (Pozniac & Dunbar 1997; Dunbar et al. 1994). Charge transfer and condensation reactions (insertion of Si between C and H with loss of H) were found to compete efficiently with the association process. The results obtained in these low-pressure conditions can be a good indication of the processes that are likely to occur in the ISM; that is to say, [SiPAH]+ complexes can be formed efficiently and play a role in the chemistry there.
Our work aims at investigating the possible relevance of [SiPAH]+ complexes in the ISM, emphasizing two aspects: the stability of these systems and the influence of the coordination of Si on the IR spectra of PAHs. We report extensive calculations of structural, thermodynamic, and mid-infrared spectroscopic properties of ground-state [SiPAH]+ complexes for four compact PAHs of increasing size: naphthalene C10H8, pyrene C16H10, coronene C24H12 and ovalene C32H14. Using the methods described in Sect. 2, we present the Si-PAH+ binding energies and structures in Sect. 3.1, and the IR calculated spectra in Sect. 3.2 where systematic effects on band intensities and positions are also discussed. In Sect. 4, the astrophysical implications are discussed and our main results are summarized in Sect. 5.
2 Methods
Geometry optimizations and frequency calculations were performed using well-established quantum chemical techniques in the framework of density functional theory (DFT, Jones & Gunnarsson 1989) with the G AUSSIAN03 package (Frisch et al. 2003).The hybrid B3LYP functional was chosen for its wide use in the study of the ground-state properties of PAHs and related molecules (see for instance Bauschlicher et al. 2008; Langhoff 1996; Bauschlicher 2002). The choice of the D95 basis set, augmented by a complete set of diffuse and polarization functions, hereafter indicated by D95++**, was made after a comparative study with other basis sets and different combinations of diffuse and polarization functions. Geometry optimizations were systematically followed by the full vibrational analyses to confirm that the geometries are minima on the potential energy surface. These were also used to determine the discrete IR spectra in the harmonic approximation.
Errors in the calculated values of the binding energies can come from uncertainties inherent in DFT methods and the finite character of the basis set. To give some insight into the relevance of the DFT choice and especially of the functional and the basis set choices to calculate binding energies, some test calculations were performed on the ionization potentials (![]() s) of Si and PAHs (cf. Table 1 here below). The calculated values of the
s) of Si and PAHs (cf. Table 1 here below). The calculated values of the ![]() s were found to be lower than the experimental ones. For PAHs, the average of this discrepancy is about -0.26 eV (from -0.20 to -0.33 eV) for vertical
s were found to be lower than the experimental ones. For PAHs, the average of this discrepancy is about -0.26 eV (from -0.20 to -0.33 eV) for vertical ![]() s. This level of calculation provides the right relative order compared to experimental data, with
s. This level of calculation provides the right relative order compared to experimental data, with ![]() s systematically underestimated by 4-5%, which is accurate enough for our purpose.
s systematically underestimated by 4-5%, which is accurate enough for our purpose.
In the study of the vibrational properties of [SiPAH]+ complexes, scaling factors have to be applied to the calculated harmonic frequencies at the DFT level for basis set incompleteness and anharmonicity effects. We determined the scaling factors for PAH0 by comparing the calculated spectrum of each PAH0 to the experimental spectrum recorded in a cold matrix of argon for naphthalene (Hudgins & Sandford 1998) and in a cryogenic matrix of neon for pyrene, coronene, and ovalene (Joblin et al. 1994). We subsequently applied a scaling factor derived from the average of the scaling factors determined for each PAH to the calculated spectra of PAH+ and the related [SiPAH]+ complexes in each spectral region of interest. Three scaling factors were used, one for the 10-15 ![]() m (1000-700 cm-1) range, one for the 5-10
m (1000-700 cm-1) range, one for the 5-10 ![]() m (2000-1000 cm-1) range, and one for the 3
m (2000-1000 cm-1) range, and one for the 3 ![]() m (3300 cm-1) C-H stretch range where modes of higher anharmonicity are present.
m (3300 cm-1) C-H stretch range where modes of higher anharmonicity are present.
Table 1:
Computational and experimental ionization potentials (![]() s) of the four considered PAHs and of Si, with computational values obtained at the B3LYP/D95++** level of theory.
s) of the four considered PAHs and of Si, with computational values obtained at the B3LYP/D95++** level of theory.
3 Results
3.1 Thermodynamics and structures
We present in this part the thermodynamic and structural properties of [SiPAH]+ complexes, completed with the thermodynamic properties of [SiPAH]0 complexes. The calculated relative enthalpies at 0 K (![]() (0 K)) of the lowest energy isomers of [SiPAH]0 and [SiPAH]+, with respect to the most stable dissociation products (Si(3P) + PAH) and (Si(3P) + PAH+) are reported in Table 2. Binding energies (
(0 K)) of the lowest energy isomers of [SiPAH]0 and [SiPAH]+, with respect to the most stable dissociation products (Si(3P) + PAH) and (Si(3P) + PAH+) are reported in Table 2. Binding energies (![]() )
correspond to
)
correspond to ![]() (0 K) values. A structure is considered stable when its
(0 K) values. A structure is considered stable when its ![]() is positive. In the case of the cationic complexes, the relative enthalpies of the reaction Si+(2P) + PAH
is positive. In the case of the cationic complexes, the relative enthalpies of the reaction Si+(2P) + PAH ![]() [SiPAH]+ at 0 K (
[SiPAH]+ at 0 K (![]() (0 K)) are also reported.
(0 K)) are also reported.
![\begin{figure}
\par\includegraphics[width=12.7cm,clip]{0863fig1.eps}
\end{figure}](/articles/aa/full_html/2009/06/aa10863-08/Timg14.gif) |
Figure 1: Lowest energy stable structures for the cationic complexes of Si with naphthalene C10H8 (structure A), pyrene C16H10 (structures B, C), coronene C24H12 (structure D), and ovalene C32H12 (structures E, F, G), optimized at the B3LYP/D95++** level of theory. The values expressed in Å indicated on each structure correspond to the Si-C bond lengths. |
| Open with DEXTER | |
Table 2:
Electronic states and calculated relative enthalpies at 0 K of the lowest energy isomers of [SiPAH]0/+, with respect to the most stable dissociation products (Si(3P) + PAH) and (Si(3P) + PAH+) (corresponding to ![]() (0 K)) and with respect to (Si+(2P) + PAH) (corresponding to
(0 K)) and with respect to (Si+(2P) + PAH) (corresponding to ![]() (0 K)) at the B3LYP/D95++** level of theory.
(0 K)) at the B3LYP/D95++** level of theory. ![]() (Si-PAH0/+) =
(Si-PAH0/+) = ![]() (0 K).
(0 K).
All [SiPAH]0/+ complexes were found to have positive ![]() ,
providing evidence of their thermodynamic stability. For the [SiPAH]0 complexes, the
,
providing evidence of their thermodynamic stability. For the [SiPAH]0 complexes, the ![]() of the most stable structures were found to lie between 0.49 and 0.58 eV, leading to an average value of
of the most stable structures were found to lie between 0.49 and 0.58 eV, leading to an average value of ![]() 0.5 eV. They were found to be in a doublet spin-state. For the [SiPAH]+ complexes, the
0.5 eV. They were found to be in a doublet spin-state. For the [SiPAH]+ complexes, the ![]() of the most stable structures, found to be in a triplet spin-state, decreases from 2.67 eV to 1.69 eV as the size of the PAH increases. Interestingly,
of the most stable structures, found to be in a triplet spin-state, decreases from 2.67 eV to 1.69 eV as the size of the PAH increases. Interestingly, ![]() decreases when
decreases when
![]() increases. This is consistent with the covalent nature of a bond, assuming the same overlap between the two orbitals in interaction. When
increases. This is consistent with the covalent nature of a bond, assuming the same overlap between the two orbitals in interaction. When
![]() is equal to zero,
is equal to zero, ![]() is maximal. This is the case for [SiC10H8]+ (
is maximal. This is the case for [SiC10H8]+ (
![]() (exp) = -0.01 eV). As the
(exp) = -0.01 eV). As the ![]() of PAH decreases roughly exponentially with the size, approaching a value of 6.0 eV, the value of
of PAH decreases roughly exponentially with the size, approaching a value of 6.0 eV, the value of ![]() is expected to decrease to a value of roughly 1.5 eV for larger species. An examination of the Mulliken charges on the Si atom and the PAH molecule shows for the larger species that the positive charge is mainly located on the PAH. This is consistent with the
is expected to decrease to a value of roughly 1.5 eV for larger species. An examination of the Mulliken charges on the Si atom and the PAH molecule shows for the larger species that the positive charge is mainly located on the PAH. This is consistent with the
![]() values.
values.
In the case of the formation of a [SiPAH]+ molecule from an Si+ and a PAH, we determined that that is an exothermic process liberating more than 3.0 eV on average.
All possible ![]() -interaction sites of Si with PAHs were considered to find the lowest-energy isomers. Their structures are displayed in Fig. 1. We found two low-energy isomers for [SiC16H10]+ and three low-energy isomers for [SiC32H14]+ with very close binding energies: 2.02 and 1.86 eV for the two isomers of [SiC16H10]+ and 1.69, 1.58, and 1.57 eV for the three isomers of [SiC32H14]+.
-interaction sites of Si with PAHs were considered to find the lowest-energy isomers. Their structures are displayed in Fig. 1. We found two low-energy isomers for [SiC16H10]+ and three low-energy isomers for [SiC32H14]+ with very close binding energies: 2.02 and 1.86 eV for the two isomers of [SiC16H10]+ and 1.69, 1.58, and 1.57 eV for the three isomers of [SiC32H14]+.
In all the most stable optimized structures Si tends to coordinate on the edge of the PAH, mostly in a two closest neighbor C-atom configuration for neutral species and in a three closest neighbor C-atom configuration for cationic species (the so-called ![]() 2 and
2 and ![]() 3 edge coordination, respectively). Interestingly, the
3 edge coordination, respectively). Interestingly, the ![]() 2 coordination mode was also found in the case of [FePAH]0 complexes (Simon & Joblin 2007) and [CuPAH]+ complexes (Dunbar 2002), and the
2 coordination mode was also found in the case of [FePAH]0 complexes (Simon & Joblin 2007) and [CuPAH]+ complexes (Dunbar 2002), and the ![]() 3 coordination mode in the case of [SiC6H6]+ complex (Jaeger et al. 2005). These coordination modes imply both a curvature of the edge of the C-skeleton and ring distortions, which had been already observed in the case of [TM-C6H6]+ (TM: transition metals) calculated structures (Jaeger et al. 2004).
3 coordination mode in the case of [SiC6H6]+ complex (Jaeger et al. 2005). These coordination modes imply both a curvature of the edge of the C-skeleton and ring distortions, which had been already observed in the case of [TM-C6H6]+ (TM: transition metals) calculated structures (Jaeger et al. 2004).
Not only Si is mainly present in its ionized form in the gas-phase of photodissociation regions (PDRs) where PAH0/+ are largely detected, but also the cationic complexed species are more interesting to study because of their higher stability. Therefore, only the spectral features of the latter species and their astrophysical implications are discussed in the following sections.
3.2 Influence of the coordination of silicon on the IR spectra of PAH+
As mentioned in Sect. 1, much work has been devoted to identifying the carriers of the astronomical AIBs. The main bands are located at 3.3, 6.2, 7.7, 8.6, 11.2, and 12.7 ![]() m. The 3.3
m. The 3.3 ![]() m band is attributed to the C-H stretch (
m band is attributed to the C-H stretch (![]()
![]() ), the 6.2
), the 6.2 ![]() m band to pure C-C stretches (
m band to pure C-C stretches (![]()
![]() ), the 7.7
), the 7.7 ![]() m band to a mixture of C-C stretches and in-plane C-H bends, the 8.6
m band to a mixture of C-C stretches and in-plane C-H bends, the 8.6 ![]() m band to pure in-plane C-H bends (
m band to pure in-plane C-H bends (![]()
![]() ). The 11.2 and 12.7
). The 11.2 and 12.7 ![]() m are both assigned to out-of-plane (oop) C-H bends (
m are both assigned to out-of-plane (oop) C-H bends (![]()
![]() ). The positions of these
). The positions of these ![]()
![]() reflect the number of adjacent C-H groups on the edge of the PAH C-skeleton. A classification was established by Hony et al. (2001). The C-H groups carried by aromatic rings with no other C-H group are called solo C-H groups and lead to IR activity around the 11.2
reflect the number of adjacent C-H groups on the edge of the PAH C-skeleton. A classification was established by Hony et al. (2001). The C-H groups carried by aromatic rings with no other C-H group are called solo C-H groups and lead to IR activity around the 11.2 ![]() m AIB. Likewise, C-H groups with one and two adjacent C-H groups are respectively called duo and trio C-H groups and lead to IR activity in the range of the 12.7
m AIB. Likewise, C-H groups with one and two adjacent C-H groups are respectively called duo and trio C-H groups and lead to IR activity in the range of the 12.7 ![]() m AIB.
m AIB.
The IR calculated spectra of all stable isomers of [SiPAH]+ complexes are reported in Fig. 2 in the [3-15] ![]() m range. The spectrum of the corresponding bare PAH+ is reported at the bottom of each figure to make comparisons easier. After calibration the calculated discrete spectra are convoluted by a Lorentzian line profile with an assumed FWHM (full width at half maximum) of 0.2
m range. The spectrum of the corresponding bare PAH+ is reported at the bottom of each figure to make comparisons easier. After calibration the calculated discrete spectra are convoluted by a Lorentzian line profile with an assumed FWHM (full width at half maximum) of 0.2 ![]() m, which accounts reasonably well for the band broadening of the astronomical spectra. The
m, which accounts reasonably well for the band broadening of the astronomical spectra. The
![]() intensity ratios that are often used to analyze observed spectra are presented in Table 3, along with the relevant shifts occurring for the
intensity ratios that are often used to analyze observed spectra are presented in Table 3, along with the relevant shifts occurring for the ![]()
![]() and
and ![]()
![]() bands. We emphasize that the extracted shifts induced by the coordination of Si to the PAH depend only marginally on the spectral calibration (a maximal deviation of -0.02
bands. We emphasize that the extracted shifts induced by the coordination of Si to the PAH depend only marginally on the spectral calibration (a maximal deviation of -0.02 ![]() m without applying scaling factors).
m without applying scaling factors).
![\begin{figure}
\par\includegraphics[width=16cm,clip]{0863fig2.eps}
\end{figure}](/articles/aa/full_html/2009/06/aa10863-08/Timg20.gif) |
Figure 2:
IR spectra of the most stable structures of PAH+ and their related Si-complexes calculated at the B3LYP/D95++** level of theory. Discrete spectra are convoluted by a Lorentzian profile with an FWHM of 0.2 |
| Open with DEXTER | |
Table 3:
![]() ratios (R) for the ground states of PAH+ and their complexes with Si and the effects of the complexation on this ratio.
ratios (R) for the ground states of PAH+ and their complexes with Si and the effects of the complexation on this ratio.
As can be seen in Fig. 2, the global features of the IR spectra of [SiPAH]+ complexes remain close to those of bare PAH+. This contrasts with the effect of the coordination of Fe to PAH+ (Simon & Joblin 2007; Hudgins et al. 2005; Simon et al. 2008; Szczepanski et al. 2006) and can be understood considering that the charge remains mainly located on the PAH with respect to the values of the ![]() s of Si and PAH.
In particular, the 3.3
s of Si and PAH.
In particular, the 3.3 ![]() m band is hardly affected by the coordination of Si, in either position or intensity.
m band is hardly affected by the coordination of Si, in either position or intensity.
For the small [SiC10H8]+ complex, the
![]() ratio decreases substantially with the coordination of Si (2.92 to 0.92), whereas for the largest species, the
ratio decreases substantially with the coordination of Si (2.92 to 0.92), whereas for the largest species, the
![]() ratios remain within the same order of magnitude (2.86 to 2.57 for pyrene, 5.24 to 2.87 for coronene, 7.27 to 5.66 for ovalene; taking the average value for several isomers). A simple explanation in terms of partial charge transfer seems sufficient to account for this trend. Given that PAH+ molecules show strong features in the [6-10]
ratios remain within the same order of magnitude (2.86 to 2.57 for pyrene, 5.24 to 2.87 for coronene, 7.27 to 5.66 for ovalene; taking the average value for several isomers). A simple explanation in terms of partial charge transfer seems sufficient to account for this trend. Given that PAH+ molecules show strong features in the [6-10] ![]() m range while PAH0 do not (Langhoff 1996), the observed decrease in the
m range while PAH0 do not (Langhoff 1996), the observed decrease in the
![]() ratio with respect to the one of bare PAH+ is due to the coordination of Si that reduces the charge carried by the PAH molecule. When the size of the PAH grows, the charge remains located mainly on the PAH with respect to the values of their
ratio with respect to the one of bare PAH+ is due to the coordination of Si that reduces the charge carried by the PAH molecule. When the size of the PAH grows, the charge remains located mainly on the PAH with respect to the values of their ![]() s; therefore, the coordination of Si has a moderated effect on the total IR activity in this region, in particular for the largest species. This differs with the effect of the coordination of Fe on PAH+, which leads to a strong decrease in the
s; therefore, the coordination of Si has a moderated effect on the total IR activity in this region, in particular for the largest species. This differs with the effect of the coordination of Fe on PAH+, which leads to a strong decrease in the
![]() ratio (Simon & Joblin 2007; Simon et al. 2008).
ratio (Simon & Joblin 2007; Simon et al. 2008).
In contrast, the coordination of Si leads to significant wavelength shifts. Several new modes are activated, most of them intense in the [6-8] ![]() m range, while the activity in the [8-10]
m range, while the activity in the [8-10] ![]() m is reduced. All the corresponding vibrations in this region are initially in-plane features before complexation. Since the coordination of Si on PAH surface breaks the plane of the PAH molecule, it leads to a scattering of the molecule vibrations in many coupled modes. We point out the appearance of strong IR activities in the 6.0
m is reduced. All the corresponding vibrations in this region are initially in-plane features before complexation. Since the coordination of Si on PAH surface breaks the plane of the PAH molecule, it leads to a scattering of the molecule vibrations in many coupled modes. We point out the appearance of strong IR activities in the 6.0 ![]() m range, due to
m range, due to ![]()
![]() modes with C atoms bounded to Si. Then [SiPAH]+ show some intense
modes with C atoms bounded to Si. Then [SiPAH]+ show some intense ![]()
![]() bands while introducing an IR activity at shorter wavelengths than the band of the bare PAH+ with an apparent blueshift average value of -0.17
bands while introducing an IR activity at shorter wavelengths than the band of the bare PAH+ with an apparent blueshift average value of -0.17 ![]() m. However, the value of the shift seems to decrease when the size of the complexed PAH molecule increases, consistently with the reason that induces this shift: the larger the PAH, the less significant the break of its plane. The calculated value of -0.17
m. However, the value of the shift seems to decrease when the size of the complexed PAH molecule increases, consistently with the reason that induces this shift: the larger the PAH, the less significant the break of its plane. The calculated value of -0.17 ![]() m has therefore to be taken with caution.
m has therefore to be taken with caution.
In the [10-15] ![]() m range of
m range of ![]()
![]() modes, the coordination of Si leads to a splitting of the bands, but the total IR activity is preserved. Even though the
modes, the coordination of Si leads to a splitting of the bands, but the total IR activity is preserved. Even though the ![]()
![]() band positions depend on the number of adjacent hydrogens, which varies from four in naphthalene to one in ovalene, complex formation increases the IR activity in the shortest wavelength domain. Indeed, the coordination of Si causes a splitting of the main
band positions depend on the number of adjacent hydrogens, which varies from four in naphthalene to one in ovalene, complex formation increases the IR activity in the shortest wavelength domain. Indeed, the coordination of Si causes a splitting of the main ![]()
![]() band of PAH+ in two bands: one at lower, and the other one at larger wavelength with respect to those of PAH+. For instance, the bands at lower and larger wavelengths shift respectively towards 11.43 and 11.97
band of PAH+ in two bands: one at lower, and the other one at larger wavelength with respect to those of PAH+. For instance, the bands at lower and larger wavelengths shift respectively towards 11.43 and 11.97 ![]() m for [SiC16H10]+ (1), while the corresponding band of C16H10+ is located at 11.76
m for [SiC16H10]+ (1), while the corresponding band of C16H10+ is located at 11.76 ![]() m. The band at lower wavelengths, located in the solo domain, is due to the
m. The band at lower wavelengths, located in the solo domain, is due to the ![]()
![]() mode of the C-H bond with its motion hindered by the presence of Si. An interesting feature is that this band is intense and occurs for all [SiPAH]+ complexes with Si coordinated above an C atom bounded to an H atom (cf. Fig. 1, [SiC10H8]+, [SiC16H10]+ (1), [SiC24H12]+, [SiC32H14]+ (2) and (3)). The average shift in this band position with respect to the position of the main
mode of the C-H bond with its motion hindered by the presence of Si. An interesting feature is that this band is intense and occurs for all [SiPAH]+ complexes with Si coordinated above an C atom bounded to an H atom (cf. Fig. 1, [SiC10H8]+, [SiC16H10]+ (1), [SiC24H12]+, [SiC32H14]+ (2) and (3)). The average shift in this band position with respect to the position of the main ![]()
![]() band of the corresponding bare PAH+ is -0.22
band of the corresponding bare PAH+ is -0.22 ![]() m. Contrary to the shift observed in the 6.0
m. Contrary to the shift observed in the 6.0 ![]() m range, this phenomenon of splitting does not depend on the molecular size but only on the geometry: the coordination of Si above an C atom bounded to an H atom systematically hinders the C-H vibration. For all these isomers, this band is the most intense when its intensity is normalized to the number of involved H atoms (cf. Fig. 3). Joblin et al. (1994) pointed out from experimental spectra on neutral PAHs the four times stronger intensity of solo
m range, this phenomenon of splitting does not depend on the molecular size but only on the geometry: the coordination of Si above an C atom bounded to an H atom systematically hinders the C-H vibration. For all these isomers, this band is the most intense when its intensity is normalized to the number of involved H atoms (cf. Fig. 3). Joblin et al. (1994) pointed out from experimental spectra on neutral PAHs the four times stronger intensity of solo ![]()
![]() than other
than other ![]()
![]() ,
which is in line with our calculations. The strong intensity and the position of the new
,
which is in line with our calculations. The strong intensity and the position of the new ![]()
![]() induced by the coordination of Si are consistent with a solo character.
induced by the coordination of Si are consistent with a solo character.
![\begin{figure}
\par\includegraphics[width=9cm,clip]{0863fig3.eps}
\end{figure}](/articles/aa/full_html/2009/06/aa10863-08/Timg22.gif) |
Figure 3:
Comparison between the |
| Open with DEXTER | |
| |
Figure 4:
The IR spectrum of the Red Rectangle nebula retrieved from the ISO archive; a) the [5.8-6.8] |
| Open with DEXTER | |
4 Astrophysical implications
Our thermodynamic results show that the binding energies of [SiPAH]+ complexes are at least 1.5 eV. Since they are three times higher than those of [SiPAH]0, [SiPAH]+ complexes are more likely to survive in the conditions of the ISM than their neutral counterparts. Furthermore, the formation of [SiPAH]+ complexes by radiative association is an exothermic process of 3.0 eV without any expected activation barrier (cf. Table 2, calculated ![]() (0 K)). The formation of [SiPAH]+ could therefore occur in many regions where Si+ and PAH0 are abundant, as in PDRs (see for instance Kaufman et al. 2006). These [SiPAH]+ complexes could then represent a significant fraction of PAH-related molecules in such regions.
(0 K)). The formation of [SiPAH]+ could therefore occur in many regions where Si+ and PAH0 are abundant, as in PDRs (see for instance Kaufman et al. 2006). These [SiPAH]+ complexes could then represent a significant fraction of PAH-related molecules in such regions.
The spectral analysis of the calculated spectra of [SiPAH]+ provides insights for astronomical detection of such species. Indeed, the coordination of Si to the PAH+ surface has two main effects:
- 1.
- a strong IR activity on the blueshifted side of the bare PAH+
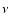
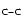 band. The corresponding shift is going from -0.30 down to -0.10
band. The corresponding shift is going from -0.30 down to -0.10 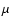 m, and seems to be decreasing when the size of the PAH increases;
m, and seems to be decreasing when the size of the PAH increases;
- 2.
- the occurrence of an additional
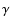
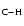 band for many isomers, blueshifted with respect to the lowest wavelength
band for many isomers, blueshifted with respect to the lowest wavelength 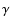
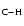 band of PAH+. The average shift is -0.2
band of PAH+. The average shift is -0.2 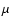 m, independent of the size of the complex but characteristic of the coordination geometry.
m, independent of the size of the complex but characteristic of the coordination geometry.
The ![]()
![]() shift induced by the coordination of Si to PAH+ that we calculated is at least -0.1
shift induced by the coordination of Si to PAH+ that we calculated is at least -0.1 ![]() m. For larger PAHs, we expect a similar shift due to the preference of Si to coordinate on the edge of the PAH, whatever its size, and then its abilility to break its plane. Therefore this value can be applied to interstellar PAHs, which are likely larger than the species we studied (Bauschlicher et al. 2008; Léger & Puget 1984). Our study provides an alternative scenario to the polycyclic aromatic nitrogen heterocycles (PANHs) explanation and the aliphatic-to-aromatic evolution scheme for the shortest wavelength position of 6.2
m. For larger PAHs, we expect a similar shift due to the preference of Si to coordinate on the edge of the PAH, whatever its size, and then its abilility to break its plane. Therefore this value can be applied to interstellar PAHs, which are likely larger than the species we studied (Bauschlicher et al. 2008; Léger & Puget 1984). Our study provides an alternative scenario to the polycyclic aromatic nitrogen heterocycles (PANHs) explanation and the aliphatic-to-aromatic evolution scheme for the shortest wavelength position of 6.2 ![]() m AIB.
m AIB.
Considering that the 11.2 ![]() m AIB is the signature of the
m AIB is the signature of the ![]()
![]() band of interstellar PAHs, we could predict a band located at 11.0
band of interstellar PAHs, we could predict a band located at 11.0 ![]() m if [SiPAH]+ complexes are present. Interestingly, such a band has been detected in many astronomical objects by the short wavelength spectrometer (SWS) onboard the infrared space observatory (ISO) (Peeters et al. 2002; Hony et al. 2001; van Diedenhoven et al. 2004). We also note that an additional band at 6.0
m if [SiPAH]+ complexes are present. Interestingly, such a band has been detected in many astronomical objects by the short wavelength spectrometer (SWS) onboard the infrared space observatory (ISO) (Peeters et al. 2002; Hony et al. 2001; van Diedenhoven et al. 2004). We also note that an additional band at 6.0 ![]() m of comparable intensity is often present. The presence of [SiPAH]+ complexes could account for this additional feature: we have seen that the
m of comparable intensity is often present. The presence of [SiPAH]+ complexes could account for this additional feature: we have seen that the ![]()
![]() shift is greater for small [SiPAH]+, and therefore these species may be the carriers of this satellite AIB. Another possibility could be larger complexes containing several Si atoms. We expect the shift to increase with the number of Si atoms, although we have not theoretically explored this effect yet.
shift is greater for small [SiPAH]+, and therefore these species may be the carriers of this satellite AIB. Another possibility could be larger complexes containing several Si atoms. We expect the shift to increase with the number of Si atoms, although we have not theoretically explored this effect yet.
Figure 4 shows the IR spectrum of the Red Rectangle, which is the brightest AIB spectrum in the sky. This region is known to be a peculiar C-rich nebula, where the low metal abundances are expected to be linked to depletion onto dust (Waelkens et al. 1996). In this object, the additional band at 6.0 ![]() m, the structure in the blue wing of the 6.2
m, the structure in the blue wing of the 6.2 ![]() m AIB and the 11.0
m AIB and the 11.0 ![]() m band are clearly visible on the spectrum retrieved from the ISO archive. The strong 7.8
m band are clearly visible on the spectrum retrieved from the ISO archive. The strong 7.8 ![]() m band observed in this object implies that large ionized PAHs exist (Bauschlicher et al. 2008; Joblin et al. 2008). The latter can offer a large surface for multiple Si atoms adsorption, which strengthens the possible assignment of the 6.0
m band observed in this object implies that large ionized PAHs exist (Bauschlicher et al. 2008; Joblin et al. 2008). The latter can offer a large surface for multiple Si atoms adsorption, which strengthens the possible assignment of the 6.0 ![]() m band to the resulting complexes.
m band to the resulting complexes.
The region of the 6.2 ![]() m AIB was fitted by three Lorentzian profiles centered at 6.0, 6.2, and 6.3
m AIB was fitted by three Lorentzian profiles centered at 6.0, 6.2, and 6.3 ![]() m after a linear continuum substraction. The integrated intensities of the 6.0 and 6.2
m after a linear continuum substraction. The integrated intensities of the 6.0 and 6.2 ![]() m bands were found to represent 13% and 22% of the total intensity, respectively. Considering that the
m bands were found to represent 13% and 22% of the total intensity, respectively. Considering that the ![]()
![]() intensity is not reduced by the coordination, 35% of PAHs would be involved in such species. Considering that the relative abundance of C in the framework of interstellar PAHs [C
intensity is not reduced by the coordination, 35% of PAHs would be involved in such species. Considering that the relative abundance of C in the framework of interstellar PAHs [C
![]() ]/[H] is equal to
]/[H] is equal to
![]() (Joblin et al. 1992) and that PAHs are constituted by an average of 80 C atoms, this leads to a relative abundance [PAH]/[H] = 8
(Joblin et al. 1992) and that PAHs are constituted by an average of 80 C atoms, this leads to a relative abundance [PAH]/[H] = 8 ![]() 10-7, in agreement with the value given by Kaufman et al. (2006). With regard to the cosmic abundance of Si ([Si]/[H] =
10-7, in agreement with the value given by Kaufman et al. (2006). With regard to the cosmic abundance of Si ([Si]/[H] =
![]() from Asplund et al. 2005), we find that the Si abundance that would be involved in these species corresponds to an order of magnitude of 1%.
from Asplund et al. 2005), we find that the Si abundance that would be involved in these species corresponds to an order of magnitude of 1%.
We compared the areas between the 11.0 and the 11.2 ![]() m bands using the same method. The area of the 11.0
m bands using the same method. The area of the 11.0 ![]() m band represents 6% of the total area composed of the 11.0 and 11.2
m band represents 6% of the total area composed of the 11.0 and 11.2 ![]() m bands. This value seems to be consistent with the higher value extracted at 6.2
m bands. This value seems to be consistent with the higher value extracted at 6.2 ![]() m taking into account that (i) a redshifted counterpart which falls in the wing of the intense 11.2
m taking into account that (i) a redshifted counterpart which falls in the wing of the intense 11.2 ![]() m AIB is expected at
m AIB is expected at ![]() 11.4
11.4 ![]() m and (ii) the coordination geometry does not lead to a systematic activation of the 11.0
m and (ii) the coordination geometry does not lead to a systematic activation of the 11.0 ![]() m vibration mode.
m vibration mode.
5 Conclusions
Tielens (1998) proposed that some Si is locked up in a dust component that differs from the silicates and whose binding energy ![]() is
is ![]() 2 eV. Our thermodynamic results show that [SiPAH]+ complexes are good candidates with a minimum binding energy
2 eV. Our thermodynamic results show that [SiPAH]+ complexes are good candidates with a minimum binding energy ![]() of 1.5 eV. The formation of such species in the ISM is expected to occur by radiative association of Si+ with a PAH0 molecule, which is an exothermic process liberating 3.0 eV.
of 1.5 eV. The formation of such species in the ISM is expected to occur by radiative association of Si+ with a PAH0 molecule, which is an exothermic process liberating 3.0 eV.
The comparison of the
![]() ratio for PAH+ and their Si-complexes illustrates the charge effect induced by the coordination of Si: this does not strongly affect the IR intensities of the spectra of PAH+. Hence the nature of Si enables the formation of cationic complexes in which the charge is mainly located on PAHs. That leads the [SiPAH]+ complexes to show IR spectra close to those of PAH+ in terms of intensities, along with characteristic features induced by the coordination of Si.
ratio for PAH+ and their Si-complexes illustrates the charge effect induced by the coordination of Si: this does not strongly affect the IR intensities of the spectra of PAH+. Hence the nature of Si enables the formation of cationic complexes in which the charge is mainly located on PAHs. That leads the [SiPAH]+ complexes to show IR spectra close to those of PAH+ in terms of intensities, along with characteristic features induced by the coordination of Si.
We propose that the presence of [SiPAH]+ species in astronomical environments can be deduced from the 11.0 ![]() m satellite band. It can also provide an explanation for the blueshifted position of the 6.2
m satellite band. It can also provide an explanation for the blueshifted position of the 6.2 ![]() m AIB. Additionally, the 6.0
m AIB. Additionally, the 6.0 ![]() m satellite band could be the signature of multiple Si-atom complexes. A firm assignment would require further experimental and theoretical work. We suggest that this identification does not require stringent constraints on the Si abundance since 1% of the cosmic Si would typically be attached to PAHs.
m satellite band could be the signature of multiple Si-atom complexes. A firm assignment would require further experimental and theoretical work. We suggest that this identification does not require stringent constraints on the Si abundance since 1% of the cosmic Si would typically be attached to PAHs.
A previous theoretical study of [FePAH]+ species has not revealed any specific fingerprints that could be used to identify these species in the ISM (Simon & Joblin 2007). Our results for [SiPAH]+ provide the first spectroscopic evidence of metal-PAH species in interstellar and circumstellar environments.
Acknowledgements
We acknowledge the anonymous referee for the fruitful comments on the manuscript, and Paolo Pilleri for his help with the ISO spectrum. This work was supported by French National Program ``Physique et Chimie du Milieu Interstellaire'' which is gratefully acknowledged. We also thank the CALMIP supercomputing facility of the Université de Toulouse where DFT calculations were performed.
References
- Allamandola, L. J., Tielens, A. G. G. M., & Barker, J. R. 1985, ApJ, 290, L25 [NASA ADS] [CrossRef]
- Asplund, M., Grevesse, N., & Sauval, A. J. 2005, in Cosmic Abundances as Records of Stellar Evolution and Nucleosynthesis, ed. T. G. Barnes, III, & F. N. Bash, ASP Conf. Ser., 336, 25 (In the text)
- Bauschlicher, Jr., C. W. 2002, ApJ, 564, 782 [NASA ADS] [CrossRef]
- Bauschlicher, Jr., C. W., Peeters, E., & Allamandola, L. J. 2008, ApJ, 678, 316 [NASA ADS] [CrossRef]
- Bohme, D. K., Wlodek, S., & Wincel, H. 1989, ApJ, 342, L91 [NASA ADS] [CrossRef] (In the text)
- Bohme, D. K., Wlodek, S., & Wincel, H. 1991, J. Am. Chem. Soc., 113, 2802 [CrossRef] (In the text)
- Dunbar, R. 2002, J. Phys. Chem. A, 106, 9809 [CrossRef] (In the text)
- Dunbar, R. C., Uechi, G. T., & Asamoto, B. 1994, J. Am. Chem. Soc., 116, 2466 [CrossRef]
- Frisch, M. J., Trucks, G. W., Schlegel, H. B., et al. 2003, Gaussian 03, Revision C.02, Gaussian, Inc., Wallingford, CT, 2004 (In the text)
- Hony, S., Van Kerckhoven, C., Peeters, E., et al. 2001, A&A, 370, 1030 [NASA ADS] [CrossRef] [EDP Sciences] (In the text)
- Hudgins, D. M., & Sandford, S. A. 1998, J. Phys. Chem. A, 102, 329 [CrossRef] (In the text)
- Hudgins, D. M., Bauschlicher, Jr., C. W., & Allamandola, L. J. 2005, ApJ, 632, 316 [NASA ADS] [CrossRef]
- Jaeger, T., van Heijnsbergen, D., Klippenstein, S., et al. 2004, J. Am. Chem. Soc., 126, 10981 [CrossRef] (In the text)
- Jaeger, J. B., Pillai, E. D., Jaeger, T. D., & Duncan, M. A. 2005, J. Phys. Chem. A, 109, 2801 [CrossRef] (In the text)
- Joblin, C., Leger, A., & Martin, P. 1992, ApJ, 393, L79 [NASA ADS] [CrossRef] (In the text)
- Joblin, C., D'Hendecourt, L., Leger, A., & Defourneau, D. 1994, A&A, 281, 923 [NASA ADS] (In the text)
- Joblin, C., Szczerba, R., Berné, O., & Szyszka, C. 2008, A&A, 490, 189 [NASA ADS] [CrossRef] [EDP Sciences]
- Jones, A. P. 2000, J. Geophys. Res., 105, 10257 [NASA ADS] [CrossRef] (In the text)
- Jones, R. O., & Gunnarsson, O. 1989, Rev. Mod. Phys., 61, 689 [NASA ADS] [CrossRef] (In the text)
- Kaufman, M. J., Wolfire, M. G., & Hollenbach, D. J. 2006, ApJ, 644, 283 [NASA ADS] [CrossRef] (In the text)
- Klotz, A., Marty, P., Boissel, P., et al. 1995, A&A, 304, 520 [NASA ADS] (In the text)
- Langhoff, S. 1996, J. Phys. Chem., 100, 2819 [CrossRef]
- Léger, A., & Puget, J. L. 1984, A&A, 137, L5 [NASA ADS]
- Peeters, E., Tielens, A. G. G. M., van Kerckhoven, C., et al. 2002, in Hot Star Workshop III: The Earliest Phases of Massive Star Birth, ed. P. Crowther, ASP Conf. Ser., 267, 403 (In the text)
- Pino, T., Dartois, E., Cao, A.-T., et al. 2008, A&A, 490, 665 [NASA ADS] [CrossRef] [EDP Sciences] (In the text)
- Pozniac, B. P., & Dunbar, R. C. 1997, J. Am. Chem. Soc., 119, 10439 [CrossRef]
- Simon, A., & Joblin, C. 2007, J. Phys. Chem. A, 111(39), 9745 (In the text)
- Simon, A., Joblin, C., Polfer, N., & Oomens, J. 2008, J. Phys. Chem. A, 112, 8551 [CrossRef]
- Srinivas, R., Hrusak, J., Sulzle, D., Bohme, D. K., & Schwarz, H. 1992, J. Am. Chem. Soc., 114, 2802 [CrossRef] (In the text)
- Szczepanski, J., Wang, H., Vala, M., et al. 2006, ApJ, 646, 666 [NASA ADS] [CrossRef]
- Tielens, A. G. G. M. 1998, ApJ, 499, 267 [NASA ADS] [CrossRef] (In the text)
- Tielens, A. G. G. M. 2008, ARA&A, 46, 289 [NASA ADS] [CrossRef] (In the text)
- van Diedenhoven, B., Peeters, E., Van Kerckhoven, C., et al. 2004, ApJ, 611, 928 [NASA ADS] [CrossRef]
- Waelkens, C., Van Winckel, H., Waters, L. B. F. M., & Bakker, E. J. 1996, A&A, 314, L17 [NASA ADS] (In the text)
- Whittet, D. C. B. 2003, Dust in the galactic environment, ed. D. C. B. Whittet (In the text)
Footnotes
- ... medium
![[*]](/icons/foot_motif.gif)
- This work is based on observations made with ISO, an ESA project with instruments funded by ESA Member States (especially the PI countries: France, Germany, The Netherlands, and the UK) and with the participation of ISAS and NASA.
All Tables
Table 1:
Computational and experimental ionization potentials (![]() s) of the four considered PAHs and of Si, with computational values obtained at the B3LYP/D95++** level of theory.
s) of the four considered PAHs and of Si, with computational values obtained at the B3LYP/D95++** level of theory.
Table 2:
Electronic states and calculated relative enthalpies at 0 K of the lowest energy isomers of [SiPAH]0/+, with respect to the most stable dissociation products (Si(3P) + PAH) and (Si(3P) + PAH+) (corresponding to ![]() (0 K)) and with respect to (Si+(2P) + PAH) (corresponding to
(0 K)) and with respect to (Si+(2P) + PAH) (corresponding to ![]() (0 K)) at the B3LYP/D95++** level of theory.
(0 K)) at the B3LYP/D95++** level of theory. ![]() (Si-PAH0/+) =
(Si-PAH0/+) = ![]() (0 K).
(0 K).
Table 3:
![]() ratios (R) for the ground states of PAH+ and their complexes with Si and the effects of the complexation on this ratio.
ratios (R) for the ground states of PAH+ and their complexes with Si and the effects of the complexation on this ratio.
All Figures
![\begin{figure}
\par\includegraphics[width=12.7cm,clip]{0863fig1.eps}
\end{figure}](/articles/aa/full_html/2009/06/aa10863-08/Timg14.gif) |
Figure 1: Lowest energy stable structures for the cationic complexes of Si with naphthalene C10H8 (structure A), pyrene C16H10 (structures B, C), coronene C24H12 (structure D), and ovalene C32H12 (structures E, F, G), optimized at the B3LYP/D95++** level of theory. The values expressed in Å indicated on each structure correspond to the Si-C bond lengths. |
| Open with DEXTER | |
| In the text | |
![\begin{figure}
\par\includegraphics[width=16cm,clip]{0863fig2.eps}
\end{figure}](/articles/aa/full_html/2009/06/aa10863-08/Timg20.gif) |
Figure 2:
IR spectra of the most stable structures of PAH+ and their related Si-complexes calculated at the B3LYP/D95++** level of theory. Discrete spectra are convoluted by a Lorentzian profile with an FWHM of 0.2 |
| Open with DEXTER | |
| In the text | |
![\begin{figure}
\par\includegraphics[width=9cm,clip]{0863fig3.eps}
\end{figure}](/articles/aa/full_html/2009/06/aa10863-08/Timg22.gif) |
Figure 3:
Comparison between the |
| Open with DEXTER | |
| In the text | |
| |
Figure 4:
The IR spectrum of the Red Rectangle nebula retrieved from the ISO archive; a) the [5.8-6.8] |
| Open with DEXTER | |
| In the text | |
Copyright ESO 2009
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


