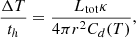| Issue |
A&A
Volume 691, November 2024
|
|
|---|---|---|
| Article Number | A256 | |
| Number of page(s) | 14 | |
| Section | Astrophysical processes | |
| DOI | https://doi.org/10.1051/0004-6361/202450013 | |
| Published online | 18 November 2024 | |
Ethanolamine ices: Experiments in simulated space conditions
1
Department of Physics, University of Trento, Via Sommarive 14, 38123 Povo, Trento, Italy
2
Italian Space Agency (ASI), Viale del Politecnico snc, 00133 Rome, Italy
3
Department of Earth Sciences, University of Florence, Via G. La Pira 4, 50121 Florence, Italy
4
INAF – Astrophysical Observatory of Arcetri, Largo E. Fermi 5, 50125 Florence, Italy
5
Centro de Astrobiologia (CAB, INTA-CSIC), 28850 Madrid, Spain
⋆ Corresponding authors; john.brucato@inaf.it; sole.biancalani@unitn.it
Received:
18
March
2024
Accepted:
16
September
2024
Context. Laboratory experiments on the interactions between complex organic molecules, interstellar dust, and ultraviolet (UV) radiation are crucial to understanding the physicochemical mechanisms that lead to the synthesis of the observed interstellar complex organic molecules (iCOMs), and to search for new molecular species not yet observed in the gas phase of the interstellar medium (ISM).
Aims. We aim to study the role of a new, recently discovered interstellar molecule, ethanolamine (EtA, NH2CH2CH2OH), in surface chemistry in the ISM.
Methods. In the laboratory, thanks to a combination of temperature programmed desorption (TPD) experiments and electron ionization (EI) mass spectrometry analyses, we studied the thermal desorption of pure ethanolamine and its mixture with water from nanometric amorphous olivine grains cooled down to 10 K, with or without UV irradiation.
Results. Ethanolamine was found to be stable, even in the presence of water, when irradiated with UV light. The presence of olivine grains strongly modified the TPD curves, trapping the molecule up to about 295 K, meaning that the precursors of some biological molecules could be retained on the grains even in the innermost parts of protoplanetary disk. We then identified a series of products formed when the molecule was irradiated onto the dust substrate.
Conclusions. Of particular interest is the fact that irradiation of ice containing ethanolamine, a molecule known to be present in the ISM, can produce more complex and astrobiologically interesting species. Furthermore, our results further our understanding of existing observational data.
Key words: astrochemistry / methods: laboratory: molecular / ISM: molecules
© The Authors 2024
 Open Access article, published by EDP Sciences, under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Open Access article, published by EDP Sciences, under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article is published in open access under the Subscribe to Open model. Subscribe to A&A to support open access publication.
1. Introduction
To date, more than 300 different molecular species have been detected in the interstellar and circumstellar media, about 130 of which are interstellar complex organic molecules (iCOMs)1. The iCOMs are chemical compounds containing six or more atoms including carbon (e.g., Blake et al. 1987; Herbst & van Dishoeck 2009; McGuire 2022). Their number is expected to increase rapidly in the coming years thanks to the large observational arrays such as ALMA and SKA, with about 15 new iCOMs being detected for the first time last year. Several space environments have been shown to host iCOMs, from molecular clouds and the earliest stages of star formation (e.g., Caselli & Ceccarelli 2012; Bacmann et al. 2012; Vastel et al. 2014) to the surroundings of high-mass and low-mass protostars, hot cores and corinos (Cazaux et al. 2003; Bottinelli et al. 2004; Jørgensen et al. 2012; Bianchi et al. 2019), and protoplanetary disks (e.g., Walsh et al. 2016; Öberg et al. 2015; Favre et al. 2018; Brunken et al. 2022). In the latter case, it is difficult to detect iCOMs because the region where the dust temperature is high enough (T > 100 K) to thermally desorb water ice and iCOMs is very small (5 au for solar-like stars; e.g., Cieza et al. 2016). In hot cores and corinos (T = 100 K–300 K), thermal desorption is expected to be dominant, while in molecular clouds, where the temperatures are low enough (10 K–20 K) to keep molecules frozen, the iCOMs may be subject to shock-induced desorption (Requena-Torres et al. 2006; Burkhardt et al. 2019), photodesorption (Öberg et al. 2010; Vastel et al. 2014; Bertin et al. 2016), or cosmic-ray-stimulated desorption (Dartois et al. 2019, 2020).
Laboratory experiments proved that iCOMs form in interstellar ice analogs through their photo- and thermal-processing (e.g., Muñoz Caro & Schutte 2003); through their energy processing with ion bombardment (e.g, Urso et al. 2020; Kaňuchová et al. 2016; Bergantini et al. 2014); or through the X-ray- (e.g., Ciaravella et al. 2019), UV-, and ion- irradiation of the sample (e.g., Gerakines et al. 2004). All these works represent an experimental verification of the efficiency of the mechanisms hypothesized as being responsible for the formation of iCOMs on the surface of the grains (e.g., Tielens & Hagen 1982; Brucato et al. 2006; Ligterink et al. 2018) or in the gas phase (e.g., Duley & Williams 1984; Charnley et al. 1995; Caselli & Ceccarelli 2012) in the interstellar medium (ISM). The catalytic role of mineral surfaces must also be taken into account. Surface catalysis is known to occur on Earth, and it is agreed that it may have played a role in increasing molecular complexity in the prebiotic era (Rimola et al. 2019). The grain-catalyzed formation of H2 in the ISM was first discussed by Gould & Salpeter (1963) and Hollenbach & Salpeter (1971) and is known to be the dominant process for H2 formation in space. Laboratory studies confirmed the role of the grains in this process (Perets et al. 2007; Vidali et al. 2009) and also in the formation of CO2 (Vidali et al. 2004; Mennella et al. 2004). It is believed that a fraction of the iCOMs are formed in space on the dust grains (Herbst & van Dishoeck 2009), so theoretical studies are being conducted to investigate the reaction pathways at the grain surface (e.g., Ahmad et al. 2020), and experiments that corroborate this hypothesis have been carried out. Strong evidence of the catalytic role of dust on low-temperature surface reactions were given by Potapov et al. (2019) and Potapov et al. (2020). Dulieu et al. (2019) proved the efficient formation of formamide on interstellar dust grain simulants. Watanabe & Kouchi (2002) produced formaldehyde and methanol via the hydrogenation of CO in H2O-CO ice at 10 K. Marchione et al. (2019) investigated the behavior of water and organic molecules on grain mantles, studying the electron-induced chemistry on the interstellar dust surface and the formation of COMs.
Ethanolamine (EtA, NH2CH2CH2OH, mass weight 61 amu) is an organic compound with the function of alcohol and amine, and it has been proposed as a precursor of phospholipids and simple amino acids such as glycine (Zhang et al. 2017) and alanine (Wirström et al. 2007). In particular, ethanolamine is the simplest phospholipid head group and forms the second most abundant phospholipid in cell membranes; its presence in the primordial Earth could therefore have stimulated the formation of phospholipids and then cell membranes (Fiore et al. 2022; Rivilla et al. 2021). Glycine (NH2CH2COOH) formation from ethanolamine was observed by Zhang et al. (2017) in simulated Archean alkaline hydrothermal vents, which are generally considered to be likely habitats for the origin of life on Earth because of their highly reactive chemical environments and possible availability of starting materials (Baross & Hoffman 1985; Martin & Russell 2006; Martín et al. 2008; Martin et al. 2014; Sojo et al. 2016). Moreover, Ehrenfreund et al. (2002) suggested that amino acids could be formed in the gas phase in an interstellar environment via the reaction between protonated amino-alcohols and formic acid (HCOOH); in such a scheme, ethanolamine would be the precursor of alanine CH3–CH(NH2)–COOH (Wirström et al. 2007). It is therefore a molecule of proven astrobiological interest.
Ethanolamine was first found in the Almahata Sitta meteorite (Glavin et al. 2010) and recently detected by Rivilla et al. (2021) in the gas phase in the ISM within the molecular cloud G + 0.693 – 0.027. It is part of the Sagittarius B2 complex, located near the galactic center, which is one of the most chemically rich areas of the Milky Way (Requena-Torres et al. 2008; Rodríguez-Almeida et al. 2021; Zeng et al. 2021; Jiménez-Serra et al. 2022; Rivilla et al. 2022a, 2023; Sanz-Novo et al. 2023). In this region, large-scale, low-velocity (< 20 km s−1) shocks (Martín et al. 2008) caused by the collision of massive molecular clouds (Zeng et al. 2020) erode the ice mantles of interstellar dust grains, resulting in an extremely rich gas-phase chemical composition. The molecular column density of ethanolamine was evaluated as N = (1.51 ± 0.07) × 1013 cm−2, and its abundance ratio to water was 3 × 10−6:1 both in the meteorite and in the ISM. So far, NH2CH2CH2OH has not been found in hot cores and hot corinos; Wirström et al. (2007) and Widicus et al. (2003) provided the upper column-density limit of 6.8 × 1013 cm−2 in the hot cores, while Nazari et al. (2024) measured it in the hot corinos, obtaining 6.0 × 1014 cm−2. This means that if the column density of ethanolamine in the hot cores and corinos were similar to that in the SgrB2 complex, the sensitivity of the observations would not be enough to provide a detection. In the near future, it may be possible to find ethanolamine even in hot cores and corinos thanks to the new generation of radio telescopes such as SKA and ngVLA. Both facilities will be able to probe the chemical composition of the ISM, hot cores and corinos, and protoplanetary disks with unprecedented sensitivity and resolution. In particular, the ngVLA’s continuous spectral coverage in the 20–50 GHz range will be essential for the detection of biogenic molecules in typical hot core sources (Selina et al. 2018). In fact, grain surface formation of ethanolamine was demonstrated by Bernstein et al. (2002) occurring by UV irradiation of a 20:2:1:1 mixture of H2O:CH3OH:NH3:HCN ices. Rivilla et al. (2021) discussed a hydrogenation chain on the surface of dust grains to form ethanolamine (based on Charnley et al. 2002), in which the precursors can be formed in the ISM according to several theoretical and experimental works (Ruaud et al. 2015; Wakelam et al. 2015; Kameneva et al. 2017; Suzuki et al. 2018; Krasnokutski 2021). More recently, Molpeceres & Rivilla (2022) also suggested an alternative formation pathway on grains. However, the detailed formation routes of ethanolamine in the ISM are still poorly understood.
Studying the solid-state chemistry of ethanolamine in the laboratory under simulated space conditions is a prerequisite to understanding whether prebiotic molecules, once formed in space, are stable enough during the protoplanetary disk phase to be delivered to planets such as Earth during their formation. In a recent study, Zhang et al. (2024) investigated the survivability of EtA in ion-, electron-, and thermally processed interstellar icy-grain mantles in cold dense clouds, as well as in KBOs in the outer Solar System. Their findings indicated that EtA is stable in the dense ISM, with a half-life of 3.6 × 107 years. In our laboratory, we conducted complementary studies on the effects of UV irradiation on ethanolamine (both pure and mixed with water), focusing on its desorption from nanometric grains of amorphous olivine, used as a dust analog. In particular, we lingered on the interaction of the molecule with UV light and olivine dust. The role of dust grains could be that of a catalyst, providing free electrons to adsorbed molecules when irradiated with UV light, which could trigger further chemical reactions. The molecules adsorbed on the active sites of the grain surface could also be spatially rearranged in a well-defined orientation to participate in stereochemical reactions (e.g., Rimola et al. 2019). In addition, the release of molecules in the gas phase due to desorption may be affected by the presence of the dust grains.
In Sect. 2, we present the experimental setup, the selection of the molecule and of amorphous olivine as a relevant cosmic dust analog, and finally the thermal desorption diagnostics. In Sect. 3, we report the results of TPD and mass spectral analyses of ethanolamine (both pure and mixed with water, with or without UV irradiation), discussing the case of ices desorbed from the bare cold finger and from the olivine dust substrate separately. In Sect. 4, we discuss the results in the context of astrophysical observations of the molecule, making the effects of the irradiation explicit, and of dust. Finally, we summarize our conclusions in Sect. 5.
2. Experimental method
2.1. Experimental set-up
The experimental apparatus consists of an ultrahigh vacuum (UHV) chamber (P ∼ 6.68 × 10−10 mbar), with feedthroughs for gas-phase deposition from a pre-chamber (P ∼ 10−7 mbar) where gas mixtures can be prepared under partial pressure control. The UHV chamber is equipped with the Hiden Analytical 3F RC 301 Pic Quadrupole Mass Spectrometer (HAL 3F RC; see Corazzi et al. 2021) and interfaces with an ARS closed-cycle helium cryocooler capable of reaching a temperature of 10 K. UV irradiation of the molecule in the 185–2000 nm wavelength range was performed using a 300 W UV-enhanced Newport xenon lamp with purified xenon at 5–20 bar connected to the UHV chamber by fiber optics. The photon flux was measured by the Optics Laboratory of the National Research Council (CNR): 2.75 × 1017 photons s−1 cm−2.
2.2. Sample selection and preparation
The ethanolamine used in this work was purchased from Alfa Aesar, with a purity of 99%+. The ices we studied were pure ethanolamine and a mixture of EtA:H2O (1:1). Although this ratio is very far from the one measured in the ISM, the results of the photochemistry indicate that the result is not particularly sensitive to the presence of water. This is further discussed in Sect. 3.
It is well known that silicates are ubiquitous in space. Dust is present in comets as observed from both ground observations with Subaru 8.2 m telescopes (e.g., Shinnaka et al. 2018; Ootsubo et al. 2020) or with 4.1 m SOAR telescopes (Picazzio et al. 2019), and from space missions, for instance, Brownlee & Stardust Mission Team (2006) for the Stardust Comet Sample Return Mission and Bockelée-Morvan et al. (2017) for the Rosetta Mission. Dust plays a major role in protoplanetary disks (e.g., Cohen & Witteborn 1985; Przygodda et al. 2003; van Boekel et al. 2004; Natta et al. 2007; Henning 2010). One of the most common silicate grains in space is olivine (Mg,Fe)2SiO4, an isomorphic mixture of forsterite (Mg2SiO4) and fayalite (Fe2SiO4; Molster et al. 2002). Olivine can be crystalline or amorphous, but the smooth profile shape of the spectral features seen in the ISM is more compatible with amorphous grains (Kraetschmer & Huffman 1979; Day 1979, 1981; Butchart & Whittet 1983). In our experiment, we used nanometric fluffy grains of amorphous olivine, which are thought to be excellent simulants of interstellar dust (Snow & Witt 1996). The grains were synthesized using a laser ablation technique in a vaporization chamber with a 10 mbar O2 oxidizing atmosphere, using an Nd-YAG solid-state UV-pulsed laser. FE-SEM analyses showed that the grains had a size of (29.4 ± 0.8) nm for small agglomerate spheres and (73 ± 12) nm for isolated spheres; the average composition was (Mg0.92Fe0.08)2SiO4 obtained by EDX probe during FE-SEM analysis. The detailed description of grain production and characterization can be found in Rotundi et al. (2002).
2.3. Thermal desorption diagnostic
Pure NH2CH2CH2OH and the 1:1 mixture of NH2CH2CH2OH and H2O were prepared in the pre-chamber. These mixtures were deposited through a valve system inside the UHV chamber, where they condensed on the cold finger cooled to 10 K. We first studied thermal desorption directly from the smooth, inert, nickel-plated bare cold finger. Then, to simulate the processes that can take place in space, we carried out the same experiments while condensing the samples on dust grains. For this purpose, the cold finger was directly covered with a thin layer of amorphous olivine grains used as a substrate. After the grain deposition, the UHV chamber was baked at 150°C for two weeks. In all measurements, during the deposition of the gas mixtures, we observed that the pressure in the UHV chamber increased from 6.68 × 10−10 mbar without ever exceeding a maximum pressure of 3 × 10−7 mbar, and this increase was proportional to the partial pressure measured in the pre-chamber. In the following sections, we report the amounts of the molecules adsorbed on the surface in terms of partial pressure, since this is a direct measure in our experimental apparatus. The condensed sample was irradiated in situ for seven hours with UV light, simulating the radiation from solar-type stars (equivalent to ∼115 days of irradiation on the surface of Mars, using the flux provided by Patel et al. 2002) and the radiation inside protoplanetary disks (equivalent to ∼220 years of irradiation at 100 au from a T Tauri star, using the flux provided by Herbig & Goodrich 1986). Secondary UV emission from cosmic rays in dense molecular clouds is also expected to be sufficient for photoprocessing (Prasad & Tarafdar 1983), but with much lower fluxes than our lamp. Padovani et al. (2024) showed that, in addition to the lines of molecular and atomic hydrogen, the CR-induced UV photon spectrum of H2 has a continuum starting at 121 nm and extending well beyond 300 nm, thus covering our lamp UV wavelengths and the typical absorption wavelengths of organic molecules. Then, the cold finger was heated at a constant rate of 1.23 K s−1 to allow the condensed molecules to desorb into the gas phase and enter the mass spectrometer for detection. This heating rate was chosen to provide a sufficiently high desorption rate for accurate mass spectrometer measurements when small amounts of molecules are deposited. We obtained the TPD curves by increasing the temperature from 10 K to 300 K, acquiring the mass spectra from 1 to 300 amu.
The TPD measurements showed that, in the case of the desorption of pure ethanolamine from the cold finger, we were in the multilayer regime (i.e., the temperature of the desorption peak increased as the coverage increased; see Fig. 1 panel A), whereas on the dust grains we were in the monolayer or sublayer regime (i.e., the temperature of the desorption peak remained constant as the coverage varied; see Fig. 1, panel B). The desorption temperature (Td) and the desorption energy (Ed) were measured by fitting the TPD curves with the Polanyi–Wigner equation (e.g., Attard & Barnes 1998) using a pre-exponential factor of 1014 s−1 (obtained by a preliminary best-fit analysis of the data and then used as a fixed parameter). A detailed description of the TPD analysis and of the determination of the desorption temperature and desorption energy was reported in our previous work (Corazzi et al. 2020). In Fig. 2 (top panel), some illustrative TPD data fits are reported.
 |
Fig. 1. TPD curves of main ethanolamine fragment (m/z 31, –CH2OH) at increasing values of partial pressure. Panel A shows the TPD curves of pure ethanolamine desorbed from the cold finger, and panel B shows the TPD curves of pure ethanolamine desorbed from the dust. |
 |
Fig. 2. Desorption from bare cold finger. Top panel: Ethanolamine main fragments TPD experimental curves (solid colored lines) and fits (dashed black lines), obtained with a partial pressure of 1.5 mbar; linear scale. Bottom panel: TPD curves for pure ethanolamine (solid lines) and for 1:1 mixture with water (dashed lines); with or without UV irradiation; all obtained at partial pressure of 0.5 mbar. |
3. Results
3.1. Desorption from the cold finger
The mass spectral analyses of pure ethanolamine we obtained were in agreement with the NIST2 mass spectrum; the only difference was that our main peak was m/z 31 instead of m/z 30, due to the difference in the ionization energy, which can vary depending on the mass spectrometer used. As can be seen in Fig. 2, the main peaks of ethanolamine molecule we followed and fitted for TPD analysis were m/z 31(-CH2OH), m/z 28 (-CH2CH2), and m/z 61 (NH2CH2CH2OH, the intact molecule). All the m/z 28 fragment TPDs showed a non-reproducible peak intensity at about 60 K due to the desorption of atmospheric N2. This is due to the co-deposition of spurious of N2 into the UHV chamber from the pre-chamber during the deposition process. As mentioned in Sect. 2.3, ethanolamine was adsorbed in a multilayer regime on the cold finger, so we were not able to obtain a unique desorption temperature, since it depends on the surface coverage and thus on the partial pressure measured in the pre-chamber. However, we obtained Td(EtA) = (196 ± 1) K at a partial pressure of 0.5 mbar and a desorption energy of Ed/kB(EtA) = (5.49 ± 0.14) ⋅ 103 K.
When pure ethanolamine was irradiated with UV light for seven hours, the mass spectrum was essentially unchanged (irradiated mass spectrum is shown by blue lines in Fig. 3). The only significant variation was observed for the water cluster (around m/z 18), whose counts decreased by more than an order of magnitude with respect to the non-irradiated case. Since we can consider the water signal as background in our instrument, its peaks are not reproducible when we do not deposit water in the UHV chamber, so this is not evidence of any change in the sample during irradiation. This result is consistent with the fact that the characterizing peaks of ethanolamine (m/z 28, 31, 61) did not show any significant change. The peak at m/z 28 was detected at a desorption temperature of about 85 K (see the bottom panel of Fig. 2), which was attributed to atmospheric N2 trapped in H2O. Upon increasing the temperature to approximately 90 K, the water undergoes a phase transition from a high-density to a low-density amorphous solid water phase, accompanied by the release of guest N2 molecules Watanabe & Kouchi (2008). Analyzing the TPD curves at a partial pressure of 0.5 mbar after UV radiation, we obtained Td(EtA) = (196 ± 1) K and Ed/kB(EtA) = (5.5 ± 0.3) ⋅ 103 K, which is consistent with the non-irradiated case. It was observed that the ethanolamine fragmentation pattern, in conjunction with its thermodynamic and kinetic parameters, remained unaltered under the UV irradiation experiments on the cold finger. Furthermore, the formation of photoproducts that were sufficiently abundant to be unambiguously detected did not occur.
 |
Fig. 3. Mass spectra comparison for the desorption of pure ethanolamine between T = 220 K and T = 240 K. |
The NH2CH2CH2OH : H2O (1:1) mixture showed mass spectra similar to those of pure ethanolamine, with a predictable increase in the m/z 18 cluster due to water. The m/z 28 TPD curve exhibited an additional peak at approximately 150 K, which was attributed to trapped N2 that desorbed simultaneously with the water ice. In this case, the TPD analyses showed an increase in desorption temperature with Td(EtA) = (201 ± 1) K due to the increase in coverage since we deposited 0.5 mbar of H2O 0.5 mbar of ethanolamine, and a desorption energy of Ed/kB(EtA) = (5.80 ± 0.16) ⋅ 103 K. We also analyzed the kinetic and thermodynamic parameters of water obtaining Td (H2O) = (160 ± 1) K and Ed/kB(H2O) = (2.85 ± 0.08) ⋅ 103 K.
When the mixture of ethanolamine and water was irradiated with UV light for seven hours, the mass spectra showed no significant variations. We then analyzed the TPD curves obtaining Td(EtA) = (200 ± 1) K and Ed/kB(EtA) = (5.7 ± 0.1) ⋅ 103 K, which is consistent with the non-irradiated case. The same 85 K and 150 K contamination peaks were found for m/z 28, which were explained in previous paragraphs of this section. Therefore, ethanolamine was found to be stable under UV irradiation, even in the presence of water. The parameters obtained for water were also in agreement with those obtained for the non-irradiated mixture: Td(H2O) = (160 ± 1) K, and Ed/kB(H2O) = (2.87 ± 0.09) ⋅ 103 K. In Fig. 2 (bottom panel), we report the main results, showing that ethanolamine and its 1:1 mixture with water had the same behavior on the bare cold finger, with or without UV irradiation.
3.2. Desorption from the dust substrate
When we compared the mass spectra of pure ethanolamine desorbed from the amorphous olivine dust (Fig. 3, green lines) with those obtained when ethanolamine desorbed from the cold finger, we saw a decrease in counts at the desorption temperature of ethanolamine and an increase in counts at higher temperatures, even if the fragmentation pattern did not change. Following m/z 28, 31 and 61, we performed TPD curve analysis for the cases at 0.5 mbar and 1.5 mbar of partial pressure. In the first case the TPD curves showed a barely visible peak at about 195 K followed by a linear increase up to about 290 K (see the dark blue line in Fig. 1, right panel, for the m/z 31 fragment and Fig. 4 for m/z 28 and m/z 31). Due to the lower counts, the m/z 61 peak was not detected. In the second case, for m/z 31 the peak at about 200 K was followed by a plateau up to about 295 K (see the light blue curve in Fig. 1, right panel, and Fig. 4). In both cases, counts returned to the baseline value after approximately five minutes at 300 K. This means that the grains retained the molecules at a higher temperature than the bare nickel-plated cold finger, so their release after the peak was gradual and prolonged. Here, we report the results for m/z 61 at 1.5 mbar of partial pressure: Td(EtA) = (200 ± 3) K, Ed/kB(EtA) = (5.5 ± 0.3) ⋅ 103 K.
 |
Fig. 4. TPD curves for m/z 28 and m/z 31 obtained with 1.5 mbar (top panels) and 0.5 mbar (bottom panels) pure ethanolamine. Comparison between the desorption from the cold finger and the desorption from the dust substrate. |
After seven hours of UV irradiation of pure ethanolamine on olivine dust, a completely different mass spectrum was observed. In Figs. 3 and 5, the mass spectra of pure ethanolamine irradiated on dust (magenta), that of pure ethanolamine irradiated on the bare cold finger (blue), that of pure ethanolamine desorbed from dust (green), and the background (black) were compared. Between 220 K and 240 K there was a significant increase in m/z 12, 16, 22, 78 peaks and m/z 41, 44, 48, 62, 65, 82, 85, 101, 119, 122 clusters, while m/z 18 and 31 decreased. For m/z 28, the peak is increased with respect to that of ethanolamine desorbed from dust without irradiation, and this is more noticeable between 180 K and 200 K (see Fig. 5). This could be due to the formation of a new molecular species with a lower desorption temperature that has an m/z 28 fragment. It is evident that the main peak characteristic of the molecule (m/z 31) has decreased; this could be due to the photodegradation of ethanolamine when irradiated on amorphous olivine dust. Together with the increase of peaks and clusters previously reported, this could mean that the UV-irradiated amorphous olivine may have had a catalytic effect on the molecule (Rimola et al. 2019). Analyzing the TPD curves of ethanolamine at 1.5 mbar partial pressure, following the representative m/z of the main clusters in addition to m/z 28, 31, 61, we obtained the result shown in Fig. 6. The main ethanolamine fragments peaked at Td = (201 ± 1) K, which is consistent with the case of non-irradiated dust. The m/z 61 peak was not observed, so we cannot report the desorption energy for the molecule. For m/z 48, we obtained a TPD curve peaked at Td(m/z 48) = (182 ± 1) K with a desorption energy of Ed/kB(m/z 48) = (3.90 ± 0.06) ⋅ 103 K, while for the other m/z values we did not obtain a clear TPD curve, but a general increase in counts between 200 K and 300 K (see the right panel of Fig. 6). This confirmed the formation of new high-m/z molecular species, whose kinetic and thermodynamic parameters could not be obtained by this analysis alone due to the low counts. However, the tentative assignments are shown in Fig. 5 and are discussed in Sect. 4.3.
 |
Fig. 5. Mass spectra comparison in three different temperature ranges: lighter shades refer to T = 180 K–200 K, medium ones to T = 220 K–240 K and darker ones to T = 260 K–280 K. Magenta shades represent ethanolamine irradiated with UV light onto the olivine dust substrate, blue ones are for ethanolamine irradiated on the clean cold finger, and green ones for ethanolamine desorbed from the olivine dust without the UV irradiation. The background, in black, is almost always negligible. Each peak has an indication of the species to which it is assigned: a formula preceded by a dash indicates a functional group (e.g., –OH); a bold formula indicates a molecular species in the m/z value associated with its molecular mass (e.g., H2O is bold in correspondence to m/z 18); a bold italics formula indicates a molecular species in correspondence to its main fragment m/z value (used only when the species has a molecular mass which does not appear in the zooms); ethanolamine is highlighted by a dashed box; all other formulae, in capital block letters, indicate the molecular species that can contribute to the peak. |
 |
Fig. 6. TPD curves of pure ethanolamine irradiated with UV light onto the olivine dust substrate. Left panel: TPD of ethanolamine main fragments and main representative of the increased peaks. Right panel: Details of the less intense peaks, at higher m/z, and molecular assignments. |
The 1:1 mixture of ethanolamine and water desorbed from the amorphous olivine dust showed a mass spectrum similar to that of pure ethanolamine desorbed from the dust substrate, with an increase in the m/z 18 cluster. Furthermore, the mass spectra showed the same behavior with respect to the temperature. We followed m/z 18, 28, 31, and 61 to perform TPD curve analysis, and obtained a desorption temperature of Td = (197 ± 3) K for the main fragments of the molecule, since m/z 61 had no visible peak. Both the curves showed a plateau following the peak up to about 300 K. Water also showed the same behavior: the peak at Td(H2O) = (158 ± 1) K and Ed/kB(H2O) = (3.04 ± 0.08) ⋅ 103 K was followed by a plateau up to about 290 K (see Fig. 7).
 |
Fig. 7. Subdivision of V883 Ori-like protoplanetary disk regions based on water phases by comparison with the desorption profile of water from the olivine dust. The violet part represents the ice region of the disk, where all the water is frozen on the dust grains, and it corresponds to the part of the TPD where desorption has not yet started. The blue part represents the snow ring, where the solid and gas phases of the water coexist, and it corresponds to the part of the TPD where most of the desorption takes place. The light gray part represents the gas region, where the water is almost all in the gas phase, and hence the final descending part of the curve. The dashed region represents the optically thick part. The blue lines correspond to the values found in Sect. 4.2, and so the 160 K/80 au line represents the classically defined snow line. |
When the same type of mixture was irradiated on the dust substrate for seven hours, the mass spectrum showed an increase in the same clusters as the irradiated pure ethanolamine (see Fig. A.1). We performed the analysis of the TPD curves and obtained for the peak of m/z 31, which represents ethanolamine’s main fragment, a desorption temperature of Td = (190 ± 6) K, which is lower than the non-irradiated case. This could be due to the large decrease in counts caused by photodissociation, which made the peak barely visible; thus, since we initially deposited only 0.5 mbar of partial pressure of ethanolamine, the fit is less accurate. This time, for m/z 48 we obtained a TPD curve peaked at Td(m/z 48) = (182 ± 1) K with a desorption energy of Ed/kB(m/z 48) = (4.08 ± 0.08) ⋅ 103 K, the latter increased with respect to the case of irradiated pure ethanolamine. Finally, water desorbed at Td(H2O) = (151 ± 1) K and Ed/kB(H2O) = (2.93 ± 0.07) ⋅ 103 K, both decreased with respect to the non-irradiated case. The profile of the TPD curve was the same, with a plateau following the peak up to 290 K. The effect of radiation on the molecule was not altered by the presence of water; this is an encouraging result, given that we have not been able to reproduce the real ratio of ethanolamine and water in the ISM, because a higher water content would saturate the mass spectrometer.
4. Discussion
4.1. Effect of UV irradiation
An initial result was that we observed no significant change in mass spectra between irradiated and non-irradiated ethanolamine. The same happened when the 1:1 mixture of ethanolamine and water was irradiated (Fig. 2, bottom panel). Thus, the kinetic and thermodynamic parameters evaluated for the molecule, both pure and mixed with water, did not show any variation with UV irradiation, demonstrating that ethanolamine is a very stable molecule under UV irradiation when deposited on the bare cold finger. In fact, in both cases, we did not observe the formation of new molecular species. To determine whether this behavior was due to a lack of molecular absorption at the emission wavelengths of the lamp, we obtained the UV absorption spectrum of EtA. Ethanolamine diluted in ethanol at room temperature showed an absorption peak at 210 nm, within the emission wavelengths of the lamp. Convolving the absorption spectrum of the molecule with the emission spectrum of the lamp, we found that the molecules were capable of absorbing 2 × 1018 photons/cm2 during the seven hours of UV irradiation. Although the ethanolamine molecules absorbed at the lamp wavelengths, our experiments did not show any dissociation or formation of new species. This could be due to a higher stability of the molecule in the solid phase at such low temperatures (10 K). Alternatively, the molecule could dissociate, but once the temperature began to rise, EtA could be reformed via a reaction between the dissociation products.
4.2. Effect of the dust substrate
A completely different situation occurs when ethanolamine interacts with olivine grains. The presence of dust significantly modified the profile of the TPD curves and their intensity, which decreased by about one order of magnitude (see Fig. 4). In the case of the deposit of 1.5 mbar partial pressure, the first peak was followed by a plateau that started to decrease at about 300 K; while in the case of 0.5 mbar, the peak was followed by a linear, albeit slow, increase that always stopped at about 300 K (see Fig. 4). Thus, the dust grains tend to retain the molecules up to much higher temperatures than the bare cold finger. This (already known) effect occurred for the crystalline grains of olivine (Corazzi et al. 2021); therefore, it is also confirmed for the amorphous olivine used in this work. In Corazzi et al. (2021), it was observed that crystalline olivine produced a second peak at higher temperatures, while our amorphous grains produced a flat region of the desorption rate. This kind of result could suggest that the mineral has a distribution of desorption energies varying from adsorption site to adsorption site (Schmid et al. 2023). Experimental (Potapov et al. 2018a,b, 2021) and theoretical (Stimpfl et al. 2006; Muralidharan et al. 2008; King et al. 2010) studies have already demonstrated the ability of silicates to strongly bind water molecules and hold them at temperatures higher than the water desorption temperature. In particular, amorphous silicates have been shown to trap water molecules up to 470 K, covering the rocky planet formation zone, with important implications for the origin of water on Earth and planet formation pathways (Potapov et al. 2024). A crucial consequence of the presence of dust grains is the need to redefine the snow lines3 in protoplanetary disks, since the molecules adsorbed on the dust grains can survive even in the innermost zones of the disks at temperatures higher than the sublimation peak. Recalling that the temperature inside a steady-state, optically thick accretion disk can be approximated by T ∝ R−3/4 (Pringle 1981) and considering that the ethanolamine molecules did not desorb completely at the first peak at about 200 K but remained adsorbed by the grain up to 295 K, the distance of the snow line from the star is reduced by about 40% for this molecule. Using the equation t = 1/kd, where kd = νne−Ed/RT (Brown & Bolina 2007), it is possible to estimate that the residence time of 20% coperture of a monolayer of ethanolamine molecules on the olivine grains (Ed/kb = (14.6 ± 0.9) × 103 K) at 200 K is about 16 Gyr. This residence time is independent of the desorption temperature; thus, it still applies with different heating rates. In fact, as explained in Collings et al. (2004) and Brown & Bolina (2007), the reduction of the heating rate affects the desorption temperature and the shape of the TPD curve peak, shifting it at lower temperatures and making it narrower. So, instead of a snow line, it would be more correct to define a snow ring; that is, a wide area of the disk where, over the average lifetime of protoplanetary disk (typically 1–3 Myr, Li & Xiao 2016), the grain does not lose its mantle completely, keeping condensed molecules at shorter heliocentric distances.
Usually, snow lines of water and iCOMs overlap in the disks and are found very close to solar-mass stars. Thus, it is difficult to resolve them spatially by observations. However, the outburst of Sun-like protostars such as V883 Ori provides an opportunity to study the sublimation regions of ices, thanks to its great luminosity that moves the snow line at larger radii (Tobin et al. 2023; Lee et al. 2019; Ruíz-Rodríguez et al. 2022; van ’t Hoff et al. 2018). Another advantage of disks around outbursting stars for our discussion is that their heating in the optically thin region is very fast. For a comparison with experimental data, estimating the heating rate of the outer disk is fundamental. To do so, we used the equations given in Johnstone et al. (2013):
where Ltot ∼ 200 L⊙ for V883 Ori, κ ∼ 400 cm2 g−1, and Cd(30 K)∼2.7 × 10−2 J g−1 K−1. At r ∼ 100 au, the heating rate is ∼40 K s−1, which is higher than our experimental value. This means that the values given in the following discussion are an extremely conservative estimate, since at higher heating rates the curves are broader and shifted at higher temperatures. In V883 Ori, Tobin et al. (2023) detected water in the disk midplane from 40 au (at smaller radii, the optically thick dust obscures the molecular line emission from the disk midplane) up to 160 au, and located the water snow line at 80 au, corresponding to the radius at which its column density begins to decrease rapidly. Knowing the laboratory desorption profile of water as reported in Fig. 7, we can locate the outer edge of the snow ring at about 110 K, beyond which almost all water molecules are iced on the surface of dust grains. The relationship between the temperature and disk radius for the outer part (> 3 au) of a V883 Ori-like disk is T ∝ R−0.53 (Labdon et al. 2021), in good agreement with isothermal flared disk relation (Kenyon & Hartmann 1987). Using this, the 80 au line corresponds to the temperature of 160 K, located just at the peak of water ice desorption, and it is thus consistent with the snow line definition. Due to the presence of the dust, the water ice desorption does continue up to 290 K, corresponding to about 25 au. This value identifies the inner edge of the snow ring, which is inside the observed optically thick region (Fig. 7); at shorter distances, the water is almost completely in the gas phase. In the same disk, using the same temperature-radius relationship, the ethanolamine snow ring should lie between about 70 au (170 K) and 25 au (295 K), with the classical snow line located at about 52 au. Unfortunately, V883 Ori has shown very low emission from N-bearing species (Lee et al. 2019; Yamato et al. 2024), so it is not a good target to detect ethanolamine. Other V883 Ori-like protoplanetary disks could be an interesting place to search for this molecule and to study its snow line.
As anticipated in Sect. 2.3, we worked in the monolayer or sublayer regime on the dust grains because of the greater adsorption surface of the dust, which means a greater number of adsorption sites. Direct comparison of the TPD curves obtained in this way with those obtained from the bare cold finger (Fig. 4) revealed that the sublimation temperature in the dust case is lower than in the bare cold finger case, and this became more pronounced as the partial pressure (and thus the coverage) increased. The real effect is the increasing of the peak’s temperature in the bare cold finger case caused by the increase of coverage in the multilayer regime.
In conclusion, the presence of dust grains alone did not produce new molecular species, but strongly modified the profile of molecular desorption curves. Since the dust has a very large surface area, it causes the ice to sublimate following the monolayer regime. This is an important aspect because with a single molecular layer it was possible to better study the effect of radiation; each molecule was in fact interacting with the radiation and with the surface of the dust grains simultaneously.
4.3. Combined effect of UV irradiation and dust substrate
As seen in Sect. 3.2, irradiation on dust grains showed a strong increase of some specific clusters. This indicated that, compared to the case of irradiation of the ethanolamine (and, similarly, of the mixture) on the cold finger, the effect of the UV radiation certainly increased when the dust was present. Using m/z 31 as the most representative peak of the ethanolamine molecule, from the comparison with the non-irradiated dust case, we can estimate that about 25% of the introduced ethanolamine must have been degraded, while the other 75% remains unaltered. The mass spectra in Figs. 3 and 5 show the formation of new species, even at high m/z, which are produced by the irradiation of ethanolamine on the olivine dust, explaining the decrease in the counts associated with ethanolamine. This is a crucial result because it makes ethanolamine a very interesting molecule from an astrobiological point of view, since we know that it is present in the ISM and that it may be a direct precursor of more complex species of even greater astrobiological interest. In fact, since it contains the most common elements constituting biomolecules (carbon C, hydrogen H, nitrogen N, and oxygen O), it could be part of the synthesis pathways of some of them. The TPD curve analysis confirmed the formation of a new species with a molecular weight equal to 48 and showed an increase in the c/s of some high m/z species between 200 K and 300 K.
The new TPD peak at m/z 48 was assigned to methanediol CH2(OH)2, which can be formed from ethanolamine. Methanediol is the simplest diol, and it has been synthesized in the laboratory by electron irradiation of the ice mixture of CH3OH and O2 (Zhu et al. 2022). Although a more complex diol, 1,2-ethenediol, has been found in the G + 0.693 − 0.027 clouds (Rivilla et al. 2022a), methanediol is still an elusive molecule in the ISM. This is probably due to the fact that its rotational spectroscopy is not yet available, and its dipole moment is too small to be detected (Barrientos et al. 2014).
Indeed, the irradiation of ethanolamine on dust could produce very reactive radicals, such as NH2, OH, CH2, and CH2OH; therefore, as suggested by Zhu et al. (2022),
CH2OH + OH → CH2(OH)2 ΔR G = −406 kJ mol−1.
This is an exoenergetic reaction leading to the formation of methanediol. Using the same radicals, it would also be possible to form the CH3OOH isomer (methyl peroxide), but this would require more reactions (Zhu et al. 2022):
CH2OH + H → CH3OH ΔR G = −396 kJ mol−1,
CH3OH → CH3O + H ΔR G = −435 kJ mol−1,
CH3O + OH → CH3OOH ΔR G = −178 kJ mol−1.
So, we are left with a series of reactions that can all be considered exoenergetic, but much more complex than the previous one, and therefore much less likely because of the experimental conditions of the system.
The other assignments were done using the mass spectra (see Fig. 5). In detail, a maximum increase of m/z 12 and m/z 16 by about an order of magnitude was observed between 180 K and 200 K compared to the cases without dust and without irradiation. This increase can be related to the fragmentation of the newly formed molecules; the signal of m/z 12 has been assigned to carbon C, which could be the result of fragmentation of different molecules containing it, but since its increase was greatest around the desorption temperature of m/z 48 it could be one of methanediol’s fragments. Also, m/z 16, which can be attributed to the presence of atomic oxygen O or the amino group NH2, showed the greatest increase around the desorption temperature of m/z 48, so it is more likely to be oxygen coming from the methanediol. The m/z 17 species showed a slight increase in c/s between 180 K and 200 K, with respect to both the cases without UV irradiation and without dust: its peak was assigned to the hydroxyl group. Water total counts (m/z 18) were almost halved with respect to the measurement on the cold finger; however, we can consider the water signal as background in our instrument, so its peaks are not reproducible when we do not deposit water in the UHV chamber. Then, m/z 22 showed an increase of a factor 14 when ethanolamine was irradiated on dust and was associated with the fragmentation of the OCO group. The greatest increase was observed around the desorption temperature of m/z 48, so it was probably again due to methanediol’s fragmentation.
As already mentioned, the m/z 28 peak increased in the irradiated dust case compared to the non-irradiated dust case, especially between 180 K and 200 K where the counts were tripled. This could be due to the CO fragment coming from CH2(OH)2. The m/z 31 signal, instead, was diminished of about 25% compared to the non-irradiated dust case. In the same cluster of m/z 31, there is m/z 32, which also showed a count decrease. While some of the formed species may contribute to the m/z 28 peak, our analysis suggests that the m/z 31 cluster is not affected by fragmentation of other formed molecules. Thus, the decrease of 25% is only due to the photolysis of the ethanolamine during its irradiation on the olivine dust substrate.
In the m/z range 41–50, there are many important signals indicating that the formation of molecules containing some functional groups (the acetyl group COCH3 at m/z 43, COO and CONH2 for m/z 44, and the carboxyl group COOH at m/z 45) all increased if compared to the non-irradiated dust case. As we have already seen, the signal at m/z 48 can be attributed to methanediol. The same molecule deprived of a hydrogen can also explain the increase in signal at m/z 47, while m/z 46 can be attributed to the fragmentation of another molecular species, since its counts increase at higher temperatures than those of methanediol and its fragments. In particular, it is associated with the fragmentation of an ethanolamine isomer (m/z 61), which we discuss in the next paragraph. Finally, m/z 41 is a signal due to the fragmentation of a more massive molecule, which is discussed in the following paragraphs.
The next zoomed-in image in Fig. 5 shows the clusters between m/z 59 and m/z 66. Here, the m/z value associated with ethanolamine, the molecule originally introduced into the apparatus, is identified. So, the signals at m/z 61 (its molecular mass); m/z 60, when it loses a hydrogen in the mass spectrometer; and m/z 62, for a hydrogenation process can be partly attributed to ethanolamine. However, the increase in all these signals suggests that an isomerization may have taken place thanks to the irradiation of the molecule onto the dust grains; the olivine’s adsorption sites, when irradiated, may be able to modify the structure of the molecule and to rearrange it into a new form that is apparently less fragmentable in the mass spectrometer. It is hypothesized that this may be CH3ONHCH3, which in the NIST spectra shows m/z 61 as the main peak, m/z 46 as the secondary peak, and m/z 28 as the tertiary one. The signals at m/z 59 and m/z 60 could be due to the same molecule losing one or two hydrogens in the mass spectrometer, and m/z 62 could be assigned to the same molecule, after a hydrogenation. Furthermore, at m/z 65 the molecule was associated with the brute formula C4NH3, which could be cyanoallene (CH2CCHCN) as well as one of its isomers. Unfortunately, the NIST mass spectrum is not available for any of these molecules; therefore, since the fragmentation is unknown, it was only possible to attribute them to their molecular mass value (m/z 65), to their hydrogenated form (m/z 66), and to m/z 64 and m/z 63. This corresponds to C4NH3 deprived of one or two hydrogens. These isomers have already been observed in the same molecular cloud that hosts ethanolamine by Rivilla et al. (2022b) and are considered interesting for prebiotic chemistry, since the presence of unsaturated bonds allows further chemical evolution that can produce biomolecules (Rosi et al. 2018); thus, they are of fundamental astrobiological importance. Finally, fragmentation of a higher mass molecule may also contribute to the increase in counts at m/z 66.
Three molecular species were assigned between m/z 78 and m/z 85, two of which correspond to their molecular mass and one to its main fragments’ m/z. In order, m/z 82 is associated with 1–aminopyrrole, C4N2H6. This is a cyclic molecule (shown in Fig. 6) that does not have a cataloged NIST mass spectrum. However, we can hypothesize that, by fragmenting, it could lose the NH2 group outside the ring and could therefore contribute to both the m/z 16 and m/z 66 signals. It was also associated with the m/z 81 and 83 signals for the loss and gain of a hydrogen, respectively. The second assigned molecular species is C2H3N3O –also cyclic– which in the NIST mass spectrum has m/z 85 as the main peak, and it may contribute a small amount to the m/z 84 peak when it loses a hydrogen in the mass spectrometer. Another possible fragment is m/z 28 and could be partly responsible for its increase. Finally, in this m/z range, glutamic acid (C5H9NO4) was associated with its main fragment m/z 84; its molecular mass is 147 amu, but the NIST mass spectrum shows m/z 84 as the main fragment followed by m/z 41 and by m/z 28. This molecule also has a small cluster around m/z 102 which could contribute to the increase in this peak. This molecule is an amino acid belonging to the category of carboxylic acids, so the demonstration of its formation from the irradiation of ethanolamine on dust could be of fundamental importance from an astrobiology point of view. G + 0.693 – 0.027 is the only source in the ISM where three carboxylic acids have been detected: formic acid (HCOOH), acetic acid (CH3COOH), and, recently, carbonic acid (HOCOOH), which was detected for the first time by Sanz-Novo et al. (2023). In the same zoomed-in image, the increase of m/z 78 was also shown; this is due to the fragmentation of the niacinamide C6H6N2O, which is assigned to m/z 122 and also responsible for the m/z 44 peak due to the CONH2 group. This molecule is also extremely important biologically, as it is a member of the B group of vitamins and forms part of the NAD+/NADH coenzyme, which is essential for the functioning of cells.
As the mass increases, so does the complexity of the compounds that may have formed, making peak assignment increasingly difficult. For this reason, some of the higher m/z peaks were not assigned.
Our experiments showed the catalytic effect of the irradiated amorphous olivine dust, which could interact with the adsorbate through photoinduced electron transfer, as studied by Linsebigler et al. (1995) for TiO2. The oxygen-metal transfer band of olivine is generally centered in the UV (Dyar et al. 2009) and is therefore capable of absorbing the incident radiation. Minerals interacting with UV light can have a photocatalytic or photoprotective behavior depending on their composition and structures, but also depending on the molecules with which they interact; for example, olivine has a catalytic role for nucleobases and a photoprotective role for amino acids (Laurent et al. 2019 and references therein). FTIR analysis of UV-irradiated organic-olivine complexes has already shown the catalytic effect of olivine in accelerating degradation kinetics (Fornaro et al. 2013, 2018), and this was also suggested by the experimental work of Potenti et al. (2018). Therefore, in our work, irradiated olivine showed catalytic behavior in the interaction with EtA.
5. Conclusions
This experimental work allowed the study of ethanolamine in simulated space conditions at low pressures (P ∼ 6.68 × 10−10 mbar) and temperatures (T ∼ 10 K). Measurements were carried out on simulated interstellar ice made from pure ethanolamine or from its 1:1 mixture with water, adsorbed directly on the cold finger or onto a substrate of fluffy, amorphous olivine dust grains, with or without UV irradiation. The results obtained were then examined; we correlated them with each other in order to understand the effect of UV irradiation, that of the olivine dust substrate, and their combined effect.
The first measurements were aimed at characterizing the ethanolamine in our instrument: the main peaks in the mass spectrum were identified and compared with those reported by NIST; the desorption temperature and energy of the molecule were determined through the TPDs. Working in a multilayer regime, the evaluated temperatures increased with the increase of the deposited ethanolamine. In the case of the 1:1 mixture of ethanolamine and water, no particular variations were observed, so the ethanolamine maintained its fragmentation pattern and desorption profile even in the presence of water. The UV irradiation of the molecule or the mixture on the bare cold finger did not show any variations. The peaks attributed to the molecule showed no decrease, indicating that either no photodissociation had occurred or that the molecule was initially dissociated and then reformed by reaction between the products. This phenomenon was observed in both the pure ethanolamine and its mixture with water when subjected to irradiation on the cold finger. This is unusual because under UV irradiation water produces OH ions, which typically play a role in promoting degradation. Moreover, it indicates that ethanolamine, if not directly bound to the surface of the dust grains, does not undergo significant chemical modification.
The presence of dust grains alone did not modify the species found during the analysis of the desorbed material, either pure or mixed, but it drastically changed the shape of the TPD curves associated with both the molecule and water (if present). This effect is fundamental from an astrophysical point of view because it shows that molecules remain adsorbed on dust grains up to temperatures higher than their desorption temperature. In our case, when using grains of amorphous olivine, we observed a plateau or increase in counts after the peak, which then began to fall at temperatures almost 100 K higher than the desorption temperature. This implies that snow lines need to be redefined. It would in fact be more correct to define a snow ring; that is, a ring around the star whose outer edge corresponds to the temperature at which the counts start to increase and whose inner edge corresponds to the temperature of the second peak (or, in this case, at the beginning of the descent of the plateau). In this region, the temperature is sufficient to start desorbing the analyzed molecule, but it is not high enough to completely strip the grain. This is crucial for protoplanetary disks as the ice and dust present in them determines the composition of the planets formed in different regions of space. Although ethanolamine has not yet been detected in protoplanetary disks, the TPD profile associated with the desorption from the dust grains has also been observed for water. Water and simple iCOM snow lines have been thoroughly studied in the disk of the Sun-like protostar V883 Ori, and we provide an experimental explanation for the observed column density of water in the protoplanetary disk. Other outbursting disks could be interesting laboratories to study the snow lines of N-bearing species such as ethanolamine, which are lacking in V883 Ori. Moreover, the presence of dust grains significantly increased the surface on which the molecules could be adsorbed (and, hence, the number of the adsorption sites), thus obtaining the monolayer regime. Achieving a monolayer adsorption is fundamental for studying the interaction of the dust grain with the molecule, especially in the case of UV radiation. This also allowed us to unambiguously determine the desorption temperature of ethanolamine from amorphous olivine grains: Td(EtA) = (200 ± 3) K.
When ethanolamine deposited on the dust was irradiated with UV light, we observed the appearance of new peaks and an increase in previously identified peaks, which, in the absence of the grains, remained below the threshold. The counts of most of these peaks were too low to allow us to evaluate the desorption temperatures, so we assigned the molecular species using the mass spectra. The observed mass spectra suggest the formation of very complex molecular species containing functional groups typical of carboxylic acids, amides, or amines. We proposed the formation pathways for the methanediol and ethanolamine isomer CH3ONHCH3. The assignments also include molecules of great astrobiological interest: C4NH3, which is already observed in the same molecular cloud as ethanolamine and considered a possible precursor of biological molecules; glutamic acid, a proteinogenic amino acid; and niacinamide, a B vitamin constituting part of NAD+/NADH, which is a fundamental biomolecule for the cell functions acting as a coenzyme for redox reactions. If confirmed, this would prove that it is possible to build molecules of high biological interest in space from molecular precursors already identified in the ISM, and it could give a further boost to theories on the origin of life. This strong increase in signal at high m/z values suggested that great complexity could be achieved simply by irradiating the molecules on the amorphous olivine grains. This process could be facilitated by the nanometric dimensions of olivine grains, similar in size to the molecules involved, which could use the adsorption sites as active sites. The fundamental role was played not only by the shape of the grains (otherwise we would see catalysis even in the absence of irradiation), but also by UV light, which, through the photoelectric effect, extracted electrons from the olivine; this in turn induced new reactions between the molecules adsorbed on the mineral. This is of fundamental astrochemical and astrobiological interest, since the presence of ethanolamine could indicate a strong chemical enrichment of the area, mediated by the presence of UV radiation and dust.
In conclusion, the results of our UV irradiation experiments demonstrate that the presence of dust has a significant impact on the chemical evolution of ethanolamine ice, leading to the formation of high-mass species. Conversely, the formation of small-mass fragments (less than 46 amu) is not affected by the presence of dust. Although ethanolamine photodissociation occurred regardless of the presence of dust, it is evident that the formation of high-mass molecules necessitates the involvement of grains. Dust grains played a role in preventing the conversion of photodissociation products back to EtA and in catalyzing the photodissociation of EtA, thereby facilitating the formation of more complex species.
The presence of water did not change the molecular species formed; this is probably due to the fact that ethanolamine itself can release OH groups when irradiated on amorphous olivine grains. This is a crucial result because it allows us to apply the conclusions drawn in our experiment to interstellar and circumstellar ices, which contain a much higher percentage of water than our simulated ones.
These encouraging results could soon lead to the discovery of new species of great astrochemical and astrobiological interest in space. The molecules whose formation has been suggested by these experiments could indeed be detected in the gaseous phase in the ISM or in protoplanetary disks after thermal or other types of desorption (e.g., shock-induced). If this were the case, there would already be laboratory studies and data on their formation in the solid state on the surface of dust grains, starting from ethanolamine exposed to UV radiation.
From the continuously updated list provided by the CDMS molecular database: https://cdms.astro.uni-koeln.de/classic/molecules
NIST Mass Spectrometry Data Center in NIST Chemistry WebBook, NIST Standard Reference Database https://webbook.nist.gov/chemistry/
The location where the transition between ice and gas of a volatile occurs in a planetary system or protoplanetary disk (Maltagliati 2020).
Acknowledgments
We thank Dr. Davide Fedele for his fruitful exchange on the astrophysical implications of this work. The authors are also grateful to the anonymous referee for his suggestions. This work has been conducted during and with the support of the Italian national inter-university PhD program in Space Science and Technology, University of Trento. Sole Biancalani’s scholarship was financed by the ASI – Italian Space Agency. V.M.R. acknowledges support from the grant PID2022-136814NB-I00 by the Spanish Ministry of Science, Innovation and Universities/State Agency of Research MICIU/AEI/10.13039/501100011033 and by ERDF, UE; the grant RYC2020-029387-I funded by MICIU/AEI/10.13039/501100011033 and by “ESF, Investing in your future”, and from the Consejo Superior de Investigaciones Científicas (CSIC) and the Centro de Astrobiología (CAB) through the project 20225AT015 (Proyectos intramurales especiales del CSIC); and from the grant CNS2023-144464 funded by MICIU/AEI/10.13039/501100011033 and by “European Union NextGenerationEU/PRTR”.
References
- Ahmad, A., Shivani, M. A., & Tandon, P. 2020, RAA, 20, 014 [NASA ADS] [Google Scholar]
- Attard, G., & Barnes, C. 1998, Surfaces (Oxford ChemistryPrimers) [CrossRef] [Google Scholar]
- Bacmann, A., Taquet, V., Faure, A., Kahane, C., & Ceccarelli, C. 2012, A&A, 541, L12 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Baross, J. A., & Hoffman, S. E. 1985, Origins Life Evol. Biosphere, 15, 327 [CrossRef] [Google Scholar]
- Barrientos, C., Redondo, P., Martínez, H., & Largo, A. 2014, ApJ, 784, 132 [NASA ADS] [CrossRef] [Google Scholar]
- Bergantini, A., Pilling, S., Rothard, H., Boduch, P., & Andrade, D. P. P. 2014, MNRAS, 437, 2720 [CrossRef] [Google Scholar]
- Bernstein, M. P., Dworkin, J. P., Sandford, S. A., Cooper, G. W., & Allamandola, L. J. 2002, Nature, 416, 401 [Google Scholar]
- Bertin, M., Romanzin, C., Doronin, M., et al. 2016, ApJ, 817, L12 [NASA ADS] [CrossRef] [Google Scholar]
- Bianchi, E., Codella, C., Ceccarelli, C., et al. 2019, MNRAS, 483, 1850 [Google Scholar]
- Blake, G. A., Sutton, E. C., Masson, C. R., & Phillips, T. G. 1987, ApJ, 315, 621 [Google Scholar]
- Bockelée-Morvan, D., Rinaldi, G., Erard, S., et al. 2017, MNRAS, 469, S443 [CrossRef] [Google Scholar]
- Bottinelli, S., Ceccarelli, C., Lefloch, B., et al. 2004, ApJ, 615, 354 [Google Scholar]
- Brown, W. A., & Bolina, A. S. 2007, MNRAS, 374, 1006 [NASA ADS] [CrossRef] [Google Scholar]
- Brownlee, D.E., & Stardust Mission Team 2006, Am. Astron. Soc. Meeting Abstr., 209, 35.06 [NASA ADS] [Google Scholar]
- Brucato, J. R., Baratta, G. A., & Strazzulla, G. 2006, A&A, 455, 395 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Brunken, N. G. C., Booth, A. S., Leemker, M., et al. 2022, A&A, 659, A29 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Burkhardt, A. M., Shingledecker, C. N., Le Gal, R., et al. 2019, ApJ, 881, 32 [Google Scholar]
- Butchart, I., & Whittet, D. C. B. 1983, MNRAS, 202, 971 [CrossRef] [Google Scholar]
- Caselli, P., & Ceccarelli, C. 2012, A&ARv, 20, 56 [Google Scholar]
- Cazaux, S., Tielens, A. G. G. M., Ceccarelli, C., et al. 2003, ApJ, 593, L51 [CrossRef] [Google Scholar]
- Charnley, S. B., Kress, M. E., Tielens, A. G. G. M., & Millar, T. J. 1995, ApJ, 448, 232 [Google Scholar]
- Charnley, S. B., Rodgers, S. D., Butner, H. M., & Ehrenfreund, P. 2002, Earth Moon Planets, 90, 349 [NASA ADS] [CrossRef] [Google Scholar]
- Ciaravella, A., Jiménez-Escobar, A., Cecchi-Pestellini, C., et al. 2019, ApJ, 879, 21 [NASA ADS] [CrossRef] [Google Scholar]
- Cieza, L. A., Casassus, S., Tobin, J., et al. 2016, Nature, 535, 258 [Google Scholar]
- Cohen, M., & Witteborn, F. C. 1985, ApJ, 294, 345 [NASA ADS] [CrossRef] [Google Scholar]
- Collings, M. P., Anderson, M. A., Chen, R., et al. 2004, MNRAS, 354, 1133 [NASA ADS] [CrossRef] [Google Scholar]
- Corazzi, M. A., Fedele, D., Poggiali, G., & Brucato, J. R. 2020, A&A, 636, A63 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Corazzi, M. A., Brucato, J. R., Poggiali, G., et al. 2021, ApJ, 913, 128 [NASA ADS] [CrossRef] [Google Scholar]
- Dartois, E., Chabot, M., Id Barkach, T., et al. 2019, A&A, 627, A55 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Dartois, E., Chabot, M., Bacmann, A., et al. 2020, A&A, 634, A103 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Day, K. L. 1979, ApJ, 234, 158 [NASA ADS] [CrossRef] [Google Scholar]
- Day, K. L. 1981, ApJ, 246, 110 [NASA ADS] [CrossRef] [Google Scholar]
- Duley, W. W., & Williams, D. A. 1984, Nature, 311, 685 [NASA ADS] [CrossRef] [Google Scholar]
- Dulieu, F., Nguyen, T., Congiu, E., Baouche, S., & Taquet, V. 2019, MNRAS, 484, L119 [NASA ADS] [CrossRef] [Google Scholar]
- Dyar, M., Sklute, E., Menzies, O., et al. 2009, Am. Mineral., 94, 883 [NASA ADS] [CrossRef] [Google Scholar]
- Ehrenfreund, P., Irvine, W., Becker, L., et al. 2002, ESA Spec. Pub., 518, 9 [Google Scholar]
- Favre, C., Fedele, D., Semenov, D., et al. 2018, ApJ, 862, L2 [NASA ADS] [CrossRef] [Google Scholar]
- Fiore, M., Chieffo, C., Lopez, A., et al. 2022, Astrobiology, 22, 598 [NASA ADS] [CrossRef] [Google Scholar]
- Fornaro, T., Brucato, J. R., Pace, E., et al. 2013, Icarus, 226, 1068 [NASA ADS] [CrossRef] [Google Scholar]
- Fornaro, T., Boosman, A., Brucato, J. R., et al. 2018, Icarus, 313, 38 [NASA ADS] [CrossRef] [Google Scholar]
- Gerakines, P. A., Moore, M. H., & Hudson, R. L. 2004, Icarus, 170, 202 [Google Scholar]
- Glavin, D. P., Aubrey, A. D., Callahan, M. P., et al. 2010, Meteorit. Planet. Sci., 45, 1695 [NASA ADS] [CrossRef] [Google Scholar]
- Gould, R. J., & Salpeter, E. E. 1963, ApJ, 138, 393 [NASA ADS] [CrossRef] [Google Scholar]
- Henning, T. 2010, ARA&A, 48, 21 [Google Scholar]
- Herbig, G. H., & Goodrich, R. W. 1986, ApJ, 309, 294 [NASA ADS] [CrossRef] [Google Scholar]
- Herbst, E., & van Dishoeck, E. F. 2009, ARA&A, 47, 427 [NASA ADS] [CrossRef] [Google Scholar]
- Hollenbach, D., & Salpeter, E. E. 1971, ApJ, 163, 155 [NASA ADS] [CrossRef] [Google Scholar]
- Jiménez-Serra, I., Rodríguez-Almeida, L. F., Martín-Pintado, J., et al. 2022, A&A, 663, A181 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Johnstone, D., Hendricks, B., Herczeg, G. J., & Bruderer, S. 2013, ApJ, 765, 133 [Google Scholar]
- Jørgensen, J. K., Favre, C., Bisschop, S. E., et al. 2012, ApJ, 757, L4 [Google Scholar]
- Kameneva, S. V., Tyurin, D. A., & Feldman, V. I. 2017, Phys. Chem. Chem. Phys., 19, 24348 [CrossRef] [Google Scholar]
- Kaňuchová, Z., Urso, R. G., Baratta, G. A., et al. 2016, A&A, 585, A155 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Kenyon, S. J., & Hartmann, L. 1987, ApJ, 323, 714 [Google Scholar]
- King, H., Stimpfl, M., Deymier, P., et al. 2010, Earth Planet. Sci. Lett., 300, 11 [CrossRef] [Google Scholar]
- Kraetschmer, W., & Huffman, D. R. 1979, Ap&SS, 61, 195 [NASA ADS] [CrossRef] [Google Scholar]
- Krasnokutski, S. A. 2021, Low Temp. Phys., 47, 199 [NASA ADS] [CrossRef] [Google Scholar]
- Labdon, A., Kraus, S., Davies, C. L., et al. 2021, A&A, 646, A102 [EDP Sciences] [Google Scholar]
- Laurent, B., Cousins, C., Pereira, M., & Martins, Z. 2019, Icarus, 323, 33 [NASA ADS] [CrossRef] [Google Scholar]
- Lee, J.-E., Lee, S., Baek, G., et al. 2019, Nat. Astron., 3, 314 [Google Scholar]
- Li, M., & Xiao, L. 2016, ApJ, 820, 36 [NASA ADS] [CrossRef] [Google Scholar]
- Ligterink, N. F. W., Terwisscha van Scheltinga, J., Taquet, V., et al. 2018, MNRAS, 480, 3628 [Google Scholar]
- Linsebigler, A. L., Lu, G., & Yates, J. T. J. 1995, Chem. Rev., 95, 735 [CrossRef] [Google Scholar]
- Maltagliati, L. 2020, Nat. Astron., 4, 554 [NASA ADS] [CrossRef] [Google Scholar]
- Marchione, D., Rosu-Finsen, A., Taj, S., et al. 2019, ACS Earth Space Chem., 3, 1915 [NASA ADS] [CrossRef] [Google Scholar]
- Martin, W., & Russell, M. 2006, Phil. Trans. R. Soc. London Ser. B, Biol. Sci., 362, 1887 [Google Scholar]
- Martín, S., Requena-Torres, M. A., Martín-Pintado, J., & Mauersberger, R. 2008, ApJ, 678, 245 [CrossRef] [Google Scholar]
- Martin, W. F., Sousa, F. L., & Lane, N. 2014, Science, 344, 1092 [NASA ADS] [CrossRef] [Google Scholar]
- McGuire, B. A. 2022, ApJS, 259, 30 [NASA ADS] [CrossRef] [Google Scholar]
- Mennella, V., Palumbo, M. E., & Baratta, G. A. 2004, ApJ, 615, 1073 [NASA ADS] [CrossRef] [Google Scholar]
- Molpeceres, G., & Rivilla, V. M. 2022, A&A, 665, A27 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Molster, F. J., Waters, L. B. F. M., Tielens, A. G. G. M., & Barlow, M. J. 2002, A&A, 382, 184 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Muñoz Caro, G. M., & Schutte, W. A. 2003, A&A, 412, 121 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Muralidharan, K., Deymier, P., Stimpfl, M., de Leeuw, N. H., & Drake, M. J. 2008, Icarus, 198, 400 [NASA ADS] [CrossRef] [Google Scholar]
- Natta, A., Testi, L., Calvet, N., et al. 2007, in Protostars and Planets V, eds. B. Reipurth, D. Jewitt, K. Keil, et al., 767 [Google Scholar]
- Nazari, P., Cheung, J. S. Y., Asensio, J. F., et al. 2024, A&A, 686, A59 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Öberg, K. I., Bottinelli, S., Jørgensen, J. K., & van Dishoeck, E. F. 2010, ApJ, 716, 825 [Google Scholar]
- Öberg, K. I., Guzmán, V. V., Furuya, K., et al. 2015, Nature, 520, 198 [Google Scholar]
- Ootsubo, T., Kawakita, H., Shinnaka, Y., Watanabe, J.-I., & Honda, M. 2020, Icarus, 338, 113450 [Google Scholar]
- Padovani, M., Galli, D., Scarlett, L. H., et al. 2024, A&A, 682, A131 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Patel, M. R., Zarnecki, J. C., & Catling, D. C. 2002, Planet. Space Sci., 50, 915 [NASA ADS] [CrossRef] [Google Scholar]
- Perets, H. B., Lederhendler, A., Biham, O., et al. 2007, ApJ, 661, L163 [NASA ADS] [CrossRef] [Google Scholar]
- Picazzio, E., Luk’yanyk, I. V., Ivanova, O. V., et al. 2019, Icarus, 319, 58 [NASA ADS] [CrossRef] [Google Scholar]
- Potapov, A., Jäger, C., & Henning, T. 2018a, ApJ, 865, 58 [NASA ADS] [CrossRef] [Google Scholar]
- Potapov, A., Mutschke, H., Seeber, P., Henning, T., & Jäger, C. 2018b, ApJ, 861, 84 [NASA ADS] [CrossRef] [Google Scholar]
- Potapov, A., Theulé, P., Jäger, C., & Henning, T. 2019, ApJ, 878, L20 [NASA ADS] [CrossRef] [Google Scholar]
- Potapov, A., Jäger, C., & Henning, T. 2020, ApJ, 894, 110 [NASA ADS] [CrossRef] [Google Scholar]
- Potapov, A., Bouwman, J., Jäger, C., & Henning, T. 2021, Nat. Astron., 5, 78 [NASA ADS] [CrossRef] [Google Scholar]
- Potapov, A., Jäger, C., Mutschke, H., & Henning, T. 2024, ApJ, 965, 48 [NASA ADS] [CrossRef] [Google Scholar]
- Potenti, S., Manini, P., Fornaro, T., et al. 2018, ACS Earth Space Chem., 2, 977 [NASA ADS] [CrossRef] [Google Scholar]
- Prasad, S. S., & Tarafdar, S. P. 1983, ApJ, 267, 603 [Google Scholar]
- Pringle, J. E. 1981, ARA&A, 19, 137 [NASA ADS] [CrossRef] [Google Scholar]
- Przygodda, F., van Boekel, R., Àbrahàm, P., et al. 2003, A&A, 412, L43 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Requena-Torres, M. A., Martín-Pintado, J., Rodríguez-Franco, A., et al. 2006, A&A, 455, 971 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Requena-Torres, M. A., Martín-Pintado, J., Martín, S., & Morris, M. R. 2008, ApJ, 672, 352 [Google Scholar]
- Rimola, A., Sodupe, M., & Ugliengo, P. 2019, Life, 9, 10 [NASA ADS] [CrossRef] [Google Scholar]
- Rivilla, V. M., Jiménez-Serra, I., Martín-Pintado, J., et al. 2021, Proc. Natl. Acad. Sci., 118, e2101314118 [NASA ADS] [CrossRef] [Google Scholar]
- Rivilla, V. M., Colzi, L., Jiménez-Serra, I., et al. 2022a, ApJ, 929, L11 [NASA ADS] [CrossRef] [Google Scholar]
- Rivilla, V. M., Jiménez-Serra, I., Martín-Pintado, J., et al. 2022b, Front. Astron. Space Sci., 9, 876870 [NASA ADS] [CrossRef] [Google Scholar]
- Rivilla, V. M., Sanz-Novo, M., Jiménez-Serra, I., et al. 2023, ApJ, 953, L20 [NASA ADS] [CrossRef] [Google Scholar]
- Rodríguez-Almeida, L. F., Rivilla, V. M., Jiménez-Serra, I., et al. 2021, A&A, 654, L1 [CrossRef] [EDP Sciences] [Google Scholar]
- Rosi, M., Skouteris, D., Casavecchia, P., et al. 2018, in Computational Science and Its Applications - ICCSA 2018, eds. O. Gervasi, B. Murgante, S. Misra, et al. (Cham: Springer International Publishing), 773 [CrossRef] [Google Scholar]
- Rotundi, A., Brucato, J. R., Colangeli, L., et al. 2002, Meteorit. Planet. Sci., 37, 1623 [NASA ADS] [CrossRef] [Google Scholar]
- Ruaud, M., Loison, J. C., Hickson, K. M., et al. 2015, MNRAS, 447, 4004 [Google Scholar]
- Ruíz-Rodríguez, D. A., Williams, J. P., Kastner, J. H., et al. 2022, MNRAS, 515, 2646 [CrossRef] [Google Scholar]
- Sanz-Novo, M., Rivilla, V. M., Jiménez-Serra, I., et al. 2023, ApJ, 954, 3 [NASA ADS] [CrossRef] [Google Scholar]
- Schmid, M., Parkinson, G. S., & Diebold, U. 2023, ACS Phys. Chem Au, 3, 44 [CrossRef] [Google Scholar]
- Selina, R. J., Murphy, E. J., McKinnon, M., et al. 2018, SPIE Conf. Ser., 10700, 107001O [NASA ADS] [Google Scholar]
- Shinnaka, Y., Ootsubo, T., Kawakita, H., et al. 2018, AJ, 156, 242 [NASA ADS] [CrossRef] [Google Scholar]
- Snow, T. P., & Witt, A. N. 1996, ApJ, 468, L65 [NASA ADS] [CrossRef] [Google Scholar]
- Sojo, V., Herschy, B., Whicher, A., Camprubí, E., & Lane, N. 2016, Astrobiology, 16, 181 [NASA ADS] [CrossRef] [Google Scholar]
- Stimpfl, M., Walker, A., Drake, M., de Leeuw, N., & Deymier, P. 2006, J. Cryst. Growth, 294, 83 [NASA ADS] [CrossRef] [Google Scholar]
- Suzuki, T., Majumdar, L., Ohishi, M., et al. 2018, ApJ, 863, 51 [NASA ADS] [CrossRef] [Google Scholar]
- Tielens, A. G. G. M., & Hagen, W. 1982, A&A, 114, 245 [Google Scholar]
- Tobin, J. J., van’t Hoff, M. L. R., Leemker, M., et al. 2023, Nature, 615, 227 [NASA ADS] [CrossRef] [Google Scholar]
- Urso, R. G., Baklouti, D., Djouadi, Z., Pinilla-Alonso, N., & Brunetto, R. 2020, ApJ, 894, L3 [NASA ADS] [CrossRef] [Google Scholar]
- van Boekel, R., Min, M., Leinert, C., et al. 2004, Nature, 432, 479 [NASA ADS] [CrossRef] [Google Scholar]
- van ’t Hoff, M. L. R., Tobin, J. J., Trapman, L., et al. 2018, ApJ, 864, L23 [Google Scholar]
- Vastel, C., Ceccarelli, C., Lefloch, B., & Bachiller, R. 2014, ApJ, 795, L2 [NASA ADS] [CrossRef] [Google Scholar]
- Vidali, G., Roser, J. E., Manicó, G., & Pirronello, V. 2004, J. Geophys. Res. (Planets), 109, E07S14 [Google Scholar]
- Vidali, G., Li, L., Roser, J. E., & Badman, R. 2009, Adv. Space Res., 43, 1291 [NASA ADS] [CrossRef] [Google Scholar]
- Wakelam, V., Loison, J. C., Hickson, K. M., & Ruaud, M. 2015, MNRAS, 453, L48 [CrossRef] [Google Scholar]
- Walsh, C., Loomis, R. A., Öberg, K. I., et al. 2016, ApJ, 823, L10 [NASA ADS] [CrossRef] [Google Scholar]
- Watanabe, N., & Kouchi, A. 2002, ApJ, 571, L173 [Google Scholar]
- Watanabe, N., & Kouchi, A. 2008, Prog. Surf. Sci., 83, 439 [NASA ADS] [CrossRef] [Google Scholar]
- Widicus, S. L., Drouin, B. J., Dyl, K. A., & Blake, G. A. 2003, J. Mol. Spectrosc., 217, 278 [NASA ADS] [CrossRef] [Google Scholar]
- Wirström, E. S., Bergman, P., Hjalmarson, Å., & Nummelin, A. 2007, A&A, 473, 177 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Yamato, Y., Notsu, S., Aikawa, Y., et al. 2024, AJ, 167, 66 [NASA ADS] [CrossRef] [Google Scholar]
- Zeng, S., Zhang, Q., Jiménez-Serra, I., et al. 2020, MNRAS, 497, 4896 [NASA ADS] [CrossRef] [Google Scholar]
- Zeng, S., Jiménez-Serra, I., Rivilla, V. M., et al. 2021, ApJ, 920, L27 [NASA ADS] [CrossRef] [Google Scholar]
- Zhang, X., Tian, G., Gao, J., et al. 2017, Origins Life Evol. Biosphere, 47, 413 [NASA ADS] [CrossRef] [Google Scholar]
- Zhang, J., Muiña, A. T., Mifsud, D. V., et al. 2024, MNRAS, 533, 826 [NASA ADS] [CrossRef] [Google Scholar]
- Zhu, C., Kleimeier, N. F., Turner, A. M., et al. 2022, Proc. Natl. Acad. Sci. U.S.A., 119 [Google Scholar]
Appendix A: Supplementary figure
 |
Fig. A.1. Mass spectra comparison for the desorption of the 1:1 mixture of ethanolamine and water between T=220K and T=240K. |
All Figures
 |
Fig. 1. TPD curves of main ethanolamine fragment (m/z 31, –CH2OH) at increasing values of partial pressure. Panel A shows the TPD curves of pure ethanolamine desorbed from the cold finger, and panel B shows the TPD curves of pure ethanolamine desorbed from the dust. |
| In the text | |
 |
Fig. 2. Desorption from bare cold finger. Top panel: Ethanolamine main fragments TPD experimental curves (solid colored lines) and fits (dashed black lines), obtained with a partial pressure of 1.5 mbar; linear scale. Bottom panel: TPD curves for pure ethanolamine (solid lines) and for 1:1 mixture with water (dashed lines); with or without UV irradiation; all obtained at partial pressure of 0.5 mbar. |
| In the text | |
 |
Fig. 3. Mass spectra comparison for the desorption of pure ethanolamine between T = 220 K and T = 240 K. |
| In the text | |
 |
Fig. 4. TPD curves for m/z 28 and m/z 31 obtained with 1.5 mbar (top panels) and 0.5 mbar (bottom panels) pure ethanolamine. Comparison between the desorption from the cold finger and the desorption from the dust substrate. |
| In the text | |
 |
Fig. 5. Mass spectra comparison in three different temperature ranges: lighter shades refer to T = 180 K–200 K, medium ones to T = 220 K–240 K and darker ones to T = 260 K–280 K. Magenta shades represent ethanolamine irradiated with UV light onto the olivine dust substrate, blue ones are for ethanolamine irradiated on the clean cold finger, and green ones for ethanolamine desorbed from the olivine dust without the UV irradiation. The background, in black, is almost always negligible. Each peak has an indication of the species to which it is assigned: a formula preceded by a dash indicates a functional group (e.g., –OH); a bold formula indicates a molecular species in the m/z value associated with its molecular mass (e.g., H2O is bold in correspondence to m/z 18); a bold italics formula indicates a molecular species in correspondence to its main fragment m/z value (used only when the species has a molecular mass which does not appear in the zooms); ethanolamine is highlighted by a dashed box; all other formulae, in capital block letters, indicate the molecular species that can contribute to the peak. |
| In the text | |
 |
Fig. 6. TPD curves of pure ethanolamine irradiated with UV light onto the olivine dust substrate. Left panel: TPD of ethanolamine main fragments and main representative of the increased peaks. Right panel: Details of the less intense peaks, at higher m/z, and molecular assignments. |
| In the text | |
 |
Fig. 7. Subdivision of V883 Ori-like protoplanetary disk regions based on water phases by comparison with the desorption profile of water from the olivine dust. The violet part represents the ice region of the disk, where all the water is frozen on the dust grains, and it corresponds to the part of the TPD where desorption has not yet started. The blue part represents the snow ring, where the solid and gas phases of the water coexist, and it corresponds to the part of the TPD where most of the desorption takes place. The light gray part represents the gas region, where the water is almost all in the gas phase, and hence the final descending part of the curve. The dashed region represents the optically thick part. The blue lines correspond to the values found in Sect. 4.2, and so the 160 K/80 au line represents the classically defined snow line. |
| In the text | |
 |
Fig. A.1. Mass spectra comparison for the desorption of the 1:1 mixture of ethanolamine and water between T=220K and T=240K. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.