Fig. B.1
Download original image
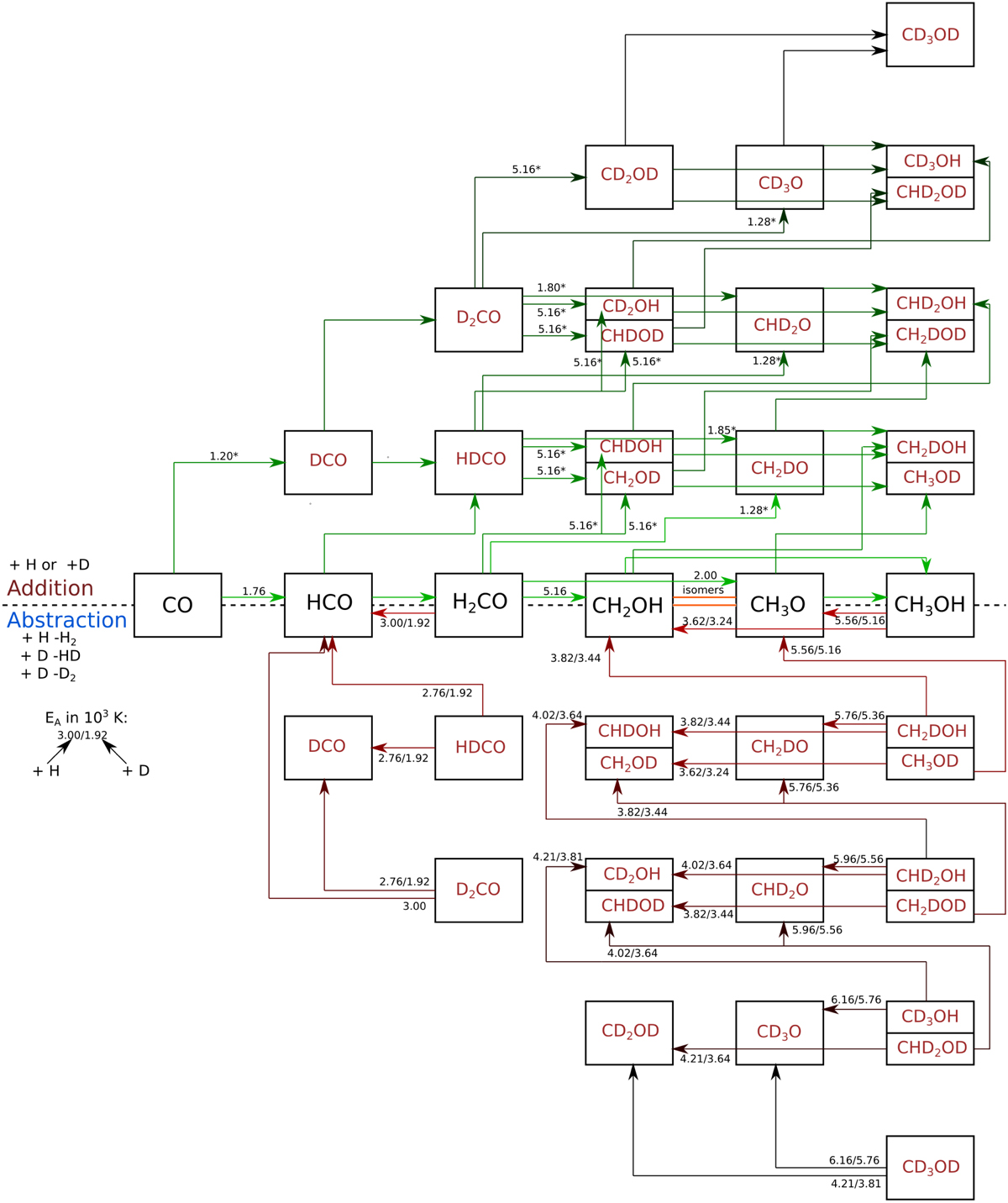
Reaction scheme for the formation of CH3OH and its deuterated isotopologues. The upper half shows the applied ‘forward reactions’ (addition reactions), either adding H (in the horizontal direction) or D (in the vertical direction). The lower half shows the employed ‘backward reactions’ (abstraction reactions), reacting with H or D and thereby removing a H2, HD, or D2 molecule. The values on top of the arrows indicate, if existing, the activation energies, EA, in 103 K. There are always two values for the abstraction reactions. The first one gives the value for the reaction with H, the second for the reaction with D. Note that our reaction scheme also includes two ‘substitution reactions’, exchanging one D atom for a H atom in H2CO and HDCO, which are not shown here for the sake of clarity.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


