Fig. C.1.
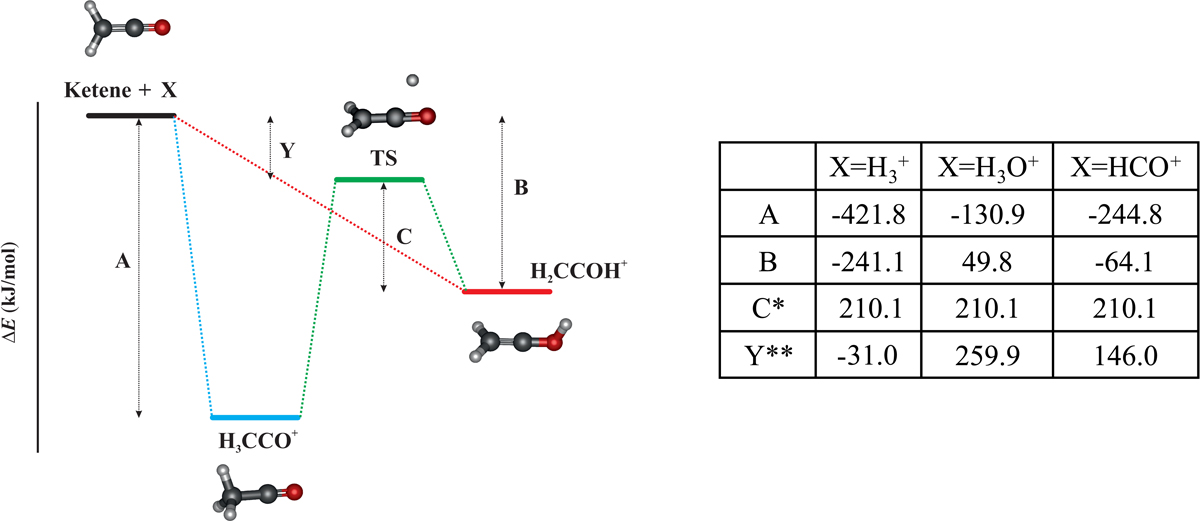
Energy diagram for the protonation of ketene. Total energies relative to those of the separated reactants, ketene and the proton donor X, are given in the enclosed table in kJ mol−1. C* is the TS energy for the interconversion between CH2COH+ and CH3CO+ isomers. Y** is the energy difference between the reactants and the interconversion TS; a negative value indicates that the TS is submerged below the reactant energy, and a positive value implies that the TS lies above the reactant energy.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


