Free Access
Table C.1
Relevant reaction rates involved in the OH+, H2O+, and H3O+ formation and destruction.
| Reaction | Rate coefficient |
| (cm3 s-1) | |
|
|
|
| H+ + O → O+ + H |
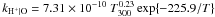
|
| O+ + H → O + H+ |
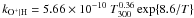
|
| O+ + H2 → OH+ + H | kO+ | H2 = 1.7 × 10-9 |
| OH+ + H2 → H2O+ + H | kOH+ | H2 = 1.01 × 10-9 |
| H2O+ + H2 → H3O+ + H | kH2O+ | H2 = 6.4 × 10-10 |
| H+ + OH → OH+ + H | kH+ | OH = 2.1 × 10-9 |
| H+ + H2O → H2O+ + H | kH+ | H2O = 6.9 × 10-9 |
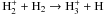
|
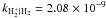
|
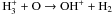
|
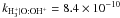
|
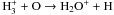
|
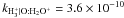
|
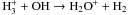
|
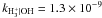
|
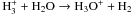
|
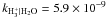
|
| OH+ + e → O + H |
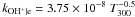
|
| H2O+ + e → products |
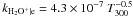
|
| H3O+ + e → products |
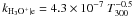
|
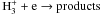
|
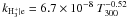
|
Notes. Rate coefficients are taken from the UMIST06 database (Woodall et al. 2007).
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


