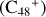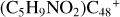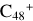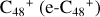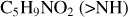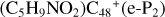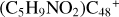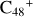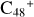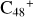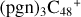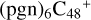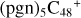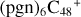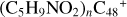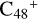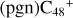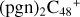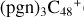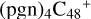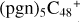| Issue |
A&A
Volume 663, July 2022
|
|
|---|---|---|
| Article Number | A52 | |
| Number of page(s) | 13 | |
| Section | Atomic, molecular, and nuclear data | |
| DOI | https://doi.org/10.1051/0004-6361/202243386 | |
| Published online | 14 July 2022 | |
Theoretical study of the formation of large, astronomically relevant PAH-organic molecule clusters
1
CAS Key Laboratory of Crust-Mantle Materials and Environment, University of Science and Technology of China,
Hefei
230026, PR China
2
CAS Center for Excellence in Comparative Planetology, University of Science and Technology of China,
Hefei
230026, PR China
e-mail: jfzhen@ustc.edu.cn
3
CAS Center for Excellence in Quantum Information and Quantum Physics, Hefei National Laboratory for Physical Sciences at the Microscale, and Department of Chemical Physics, University of Science and Technology of China,
Hefei
230026, PR China
4
CAS Key Laboratory for Research in Galaxies and Cosmology, Department of Astronomy, University of Science and Technology of China,
96 Jinzhai RD., Hefei,
Anhui
230026, PR China
Received:
21
February
2022
Accepted:
25
April
2022
Context. Polycyclic aromatic hydrocarbon (PAH) molecules play an essential role in the prebiotic compound evolution network in the interstellar medium (ISM). A recent experimental study revealed that large, astronomically relevant PAH-organic molecule clusters are gradually formed through the ion-molecule collision reaction pathway in the presence of a strong radiation field.
Aims. We present a theoretical survey for the formation processes of PAH-organic molecule clusters (e.g., such as the graphene carbon cluster (C48) organic molecule (Pyroglutaminol, pgn, C5H9NO2) cluster cations, (pgn)nC48+, n = [1,6]), to illustrate the building block mechanism for the formation of large prebiotic compounds.
Methods. To investigate the stability and the building block formation mechanisms of PAH-organic molecule clusters in the ion-molecule collision reaction process, we carried out theoretical calculations with DFT, including the hybrid density functional B3LYP, as implemented in the Gaussian 16 program. The basis set of the 6-311++G** and 6-31+G** was selected and used for different cluster systems.
Results. We investigated the structure of newly formed species and the energy for these reaction pathways. The ion-molecule reaction between ((C5H9NO2)nC48+, n = [0,5]) with C5H9NO2 readily occur, resulting in a very large number of reaction pathways and very complex newly formed molecular clusters. An expanded tree (in building block pathways) shows the trunk and branches of these various formation pathways. These clusters (e.g., the graphene carbon cluster and its organic molecules) provide a possible formation and chemical-evolution route for the large complex prebiotic compounds in bottom-up and energy allowed processes in the ISM.
Conclusions. The gas-phase reactions between large PAH species and organic molecules occur relatively easily, resulting in a very large number of reaction pathways and very complex newly formed molecular clusters. These PAH-organic molecule clusters will lead to large organic molecules, which may contain some of the critical molecular configurations that can characterize living material.
Key words: astrochemistry / molecular processes / methods: laboratory: molecular / ISM: molecules / infrared: ISM
© Y. Yang et al. 2022
 Open Access article, published by EDP Sciences, under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Open Access article, published by EDP Sciences, under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article is published in open access under the Subscribe-to-Open model. Subscribe to A&A to support open access publication.
1 Introduction
In the interstellar medium (ISM) of galaxies, the infrared (IR) broadband (on the order of 20–40 cm−1) features, at 3.3, 6.2, 7.7, 8.6, and 11.2 µm, are generally attributed to the family of poly-cyclic aromatic hydrocarbon (PAH) molecules (e.g., Sellgren 1984; Puget & Leger 1989; Allamandola et al. 1989). These PAH species are found to be ubiquitous and abundant, containing ~10% of the elemental carbon and playing an essential role in the ionization and energy balance of the ISM of galaxies (Tielens 2013, and references therein). Furthermore, PAHs can subsequently undergo physical and chemical processes for several hundred million years in the harsh environment of the ISM. As part of this evolution, PAHs may acquire some types of functional groups and functionalize, such as methyl (−CH3), vinyl (-CHCH2), methoxy (-OCH3), amino (-NH2), cyano and isocyano (-CN, -NC), acid (-COOH), and hydroxyl (-OH) (Hollenbach & Tielens 1999; Allamandola 2011). More recently, two nitrile-group–functionalized PAHs, 1- and 2-cyanonaphthalene, have been detected in the ISM in particular. Both bicyclic ring molecules were observed in the TMC-1 molecular cloud (McGuire et al. 2021).
The interstellar IR also determines the presence of PAH clusters and very small dust grain features (e.g., Van Diedenhoven et al. 2004; Rapacioli et al. 2005; Berné et al. 2007; Pilleri et al. 2015). In the molecular cloud, PAH clusters are believed to be the self-assembled intermediaries between PAHs and other coexisting molecules, and PAH radicals are discussed to be the driving force in the cluster formation process (Rapacioli et al. 2006; Rhee et al. 2007; Zhen 2019; Gavilan et al. 2020). In addition, PAH band features are also prominent in planet-forming disks around young stars (Habart et al. 2006; Doucet et al. 2007), and PAHs, as well as fullerene (C60/70). These are important components of solar system meteorites (Sephton & Botta 2008; Becker et al. 1994). Hence, understanding the processes that regulate the origin and evolution of these species and their relationship to the organic inventory of space has become a special focus in the field of astrochemistry (Tielens 2013, and references therein).
At present, more than 260 molecules have been detected in the interstellar medium or circumstellar shells of photo-dissociation regions (PDRs), and 69 molecules detected in extra-galactic sources1. And interstellar (complex organic) molecules are thought to be the building blocks of more complex pre-biotic compounds (Dulieu et al. 2019). Organic molecules are known to exist in star-forming regions and in protoplanetary disks where planets are formed (Herbst & van Dishoeck 2009, and references therein). Observations have established the existence of ethyl cyanide (CH3CH2CN), acetone (CH3COCH3), and (possibly) the amino acid glycine (NH2CH2COOH) in the interstellar clouds (Snyder 1997). (Favre et al. 2018) also reported the first detection of the simplest organic acid (formic acid, HCOOH) toward the TW Hydrae protoplanetary disk. However, free organic molecules in the gas phase are more fragile in the face of UV irradiation and cannot survive in the harsh environment of the ISM (e. g., galactic interstellar UV radiation field). As in the HI regions (5.0 < h v < 13.6 eV), the energy of a single photon is capable of dissociating these molecules (Herbst & van Dishoeck 2009; Woods et al. 2012).
As the most important carbon reservoir in interstellar space, PAH molecules and their derivatives may play as an essential role in the evolution network of prebiotic compounds in space (e.g., Puget & Leger 1989; Allamandola 2011; Tielens 2013). As host, large PAH species, PAH clusters, or very small dust grains can protect these gas-phase complex organic molecules from UV dissociation, allowing them to survive in the areas of high luminosity in interstellar environments (Zhen 2019; Gavilan et al. 2020; Hu et al. 2021a,b; Yang et al. 2021). However, the buildingblock mechanism and the chemical processes of the gas-phase free organic molecules accretes on or into the large PAH species, PAH clusters, or very small dust grains is still unclear.
In a recent contribution by (Hu et al. 2021a), the laboratory gas-phase formation of astronomically relevant large PAH and organic molecule clusters was studied. Three gas-phase reaction systems: dicoronylene (DC, C48H20)-pyroglutamic acid (Pga, C5H7NO3), DC-proline (Pro, C5H9NO2), and DC-pyroglutaminol (Pgn, C5H9NO2) were experimentally investigated. Their experiments indicate that PAH-organic molecule cluster cations (e.g., 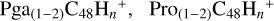 , and
, and 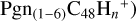 and carbon cluster-organic molecule cluster cations (e.g.,
and carbon cluster-organic molecule cluster cations (e.g., 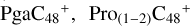 , and
, and 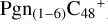 ) are formed through an ion-molecule collision reaction pathway in the presence of a strong galactic interstellar radiation field. From the experimental results, the complex organic molecules exhibit a different formation behavior when reacting with PAH species, and each functional group of the organic molecule may play a decisive role in their chemical reactivity with PAH species (Hu et al. 2021a).
) are formed through an ion-molecule collision reaction pathway in the presence of a strong galactic interstellar radiation field. From the experimental results, the complex organic molecules exhibit a different formation behavior when reacting with PAH species, and each functional group of the organic molecule may play a decisive role in their chemical reactivity with PAH species (Hu et al. 2021a).
In this work, to investigate the stability and the building block formation mechanisms of PAH and organic molecule clusters in the ion-molecule collision reaction process, we explored large PAH cations (e.g., the graphene carbon cluster, 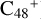 ) and organic molecules (Pyroglutaminol, C5H9NO2) as the initial precursor. We present a theoretical survey for the formation processes of PAH-organic molecule clusters (
) and organic molecules (Pyroglutaminol, C5H9NO2) as the initial precursor. We present a theoretical survey for the formation processes of PAH-organic molecule clusters (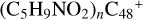 , n = [1,6]), based on an investigation of the structure of newly formed cluster species and the energy for these reaction pathways. An expanded tree (in building block pathway) shows the trunk and branches of these various formation pathways for these clusters. In addition, the IR spectra of some PAH/organic molecule clusters are also calculated and discussed. Furthermore, we note that although pyroglutaminol (C5H9NO2) has not been detected in the ISM, it does not indicate that pyrog-lutaminol does not exist at all. In addition, glutamic acid (C5H9NO4) and pyroglutamic acid (C5H9NO3) have been identified in the carbonaceous chondrites (Cooper et al. 2001; Glavin et al. 2018), and Proline (C5H9NO2) can be synthesized from glutamic acid. Based on these findings, we can make a reasonable assumption that pyroglutaminol may also exist in carbonaceous chondrites.
, n = [1,6]), based on an investigation of the structure of newly formed cluster species and the energy for these reaction pathways. An expanded tree (in building block pathway) shows the trunk and branches of these various formation pathways for these clusters. In addition, the IR spectra of some PAH/organic molecule clusters are also calculated and discussed. Furthermore, we note that although pyroglutaminol (C5H9NO2) has not been detected in the ISM, it does not indicate that pyrog-lutaminol does not exist at all. In addition, glutamic acid (C5H9NO4) and pyroglutamic acid (C5H9NO3) have been identified in the carbonaceous chondrites (Cooper et al. 2001; Glavin et al. 2018), and Proline (C5H9NO2) can be synthesized from glutamic acid. Based on these findings, we can make a reasonable assumption that pyroglutaminol may also exist in carbonaceous chondrites.
 |
Fig. 1 Molecular geometry of graphene carbon cluster cations |
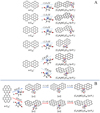 |
Fig. 2 Reaction pathways and the optimized structures of |
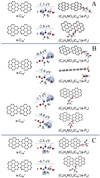 |
Fig. 3 Reaction pathways and the optimized structures of |
2 Theoretical calculation results and discussion
To understand the process of gas-phase aggregation, we illustrate the building-block mechanism for the formation of large prebiotic compounds in the gas phase, carrying out a theoretical calculation survey of the possible formation pathway of large, astronomically relevant PAH-organic molecule clusters (e.g., the graphene carbon cluster-organic molecule cluster cations, ![$\left( {{{\left( {{{\rm{C}}_{\rm{5}}}{{\rm{H}}_9}{\rm{N}}{{\rm{O}}_{\rm{2}}}} \right)}_n}{{\rm{C}}_{{\rm{48}}}}^{\rm{ + }},\,n = \left[ {1,6} \right]} \right)$](/articles/aa/full_html/2022/07/aa43386-22/aa43386-22-eq7.png) . The theoretical calculations were performed on the basis of density functional theory (DFT) with the hybrid density functional B3LYP (Becke 1992; Lee et al. 1988), as implemented in the Gaussian 16 program (Frisch et al. 2016). The basis set of the 6-311++G** and 6-31+G** was selected and used for different cluster systems, respectively. To account for the intermolecular interactions, the dispersion-correction (D3, Grimme et al. 2011) is considered for each system. All species’ geometries were optimized at the local minimum of their potential energy surface. The zero-point energy and thermal corrections were obtained from the frequency calculation to correct the molecular energy.
. The theoretical calculations were performed on the basis of density functional theory (DFT) with the hybrid density functional B3LYP (Becke 1992; Lee et al. 1988), as implemented in the Gaussian 16 program (Frisch et al. 2016). The basis set of the 6-311++G** and 6-31+G** was selected and used for different cluster systems, respectively. To account for the intermolecular interactions, the dispersion-correction (D3, Grimme et al. 2011) is considered for each system. All species’ geometries were optimized at the local minimum of their potential energy surface. The zero-point energy and thermal corrections were obtained from the frequency calculation to correct the molecular energy.
In addition, throughout the calculation process, we note that our computational strategy avoids trying to calculate all the possible molecular structures and the formation pathways, as there are hundreds of different (isomers) products that have potentially formed in our experiments, and we cannot possibly calculate all of them. In the calculation processes, we take a continuous simplification process to limit and narrow down the range and intensity of the computation as the clusters keep getting larger. Since the clusters keep getting larger, the number of those isomers produced in our experiments increases very quickly. Based on this fact, we only took a proportion of them to use as the representative isomers for all the isomers that are produced. In the process of selecting representative isomers, we gradually simplified some common or similar isomers.
The obtained calculation results are presented as follows: we start (Sect. 2.1) with the optimized geometric structure of 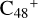 , and pyroglutaminol, C5H9NO2, as shown in Fig. 1. In Sects 2.2 and 2.3, we focus on the reaction pathways and the optimized structures of
, and pyroglutaminol, C5H9NO2, as shown in Fig. 1. In Sects 2.2 and 2.3, we focus on the reaction pathways and the optimized structures of 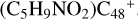 , as shown in Fig. 2 and 3. Sections 2.4 and 2.5 investigate the reaction pathways and the optimized structures of
, as shown in Fig. 2 and 3. Sections 2.4 and 2.5 investigate the reaction pathways and the optimized structures of 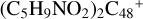 and
and 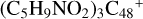 , as shown in Fig. 4; Sects. 2.6 and 2.7 focus on the reaction pathways and the optimized structures of
, as shown in Fig. 4; Sects. 2.6 and 2.7 focus on the reaction pathways and the optimized structures of 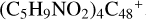 , and
, and 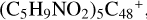 , as shown in Fig. 5. Section 2.8 presents the reaction pathways and the optimized structures of
, as shown in Fig. 5. Section 2.8 presents the reaction pathways and the optimized structures of  , as shown in Fig. 6. We end with a brief discussion, in Sect. 2.9 on an expanded tree (in building block pathways), which shows the trunk and branches of these various formation pathways for these clusters
, as shown in Fig. 6. We end with a brief discussion, in Sect. 2.9 on an expanded tree (in building block pathways), which shows the trunk and branches of these various formation pathways for these clusters 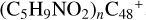 , n from 0 to 6, as shown in Fig. 7.
, n from 0 to 6, as shown in Fig. 7.
2.1 Optimized geometric structure of 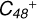 , and C5H9NO2
, and C5H9NO2
For the graphene carbon cluster C48 cation, we assume there is no carbon skeleton rearrangement during the electron impact ionization and photo-fragmentation process (Zhen et al. 2014; Zhen 2019). In this way, the molecular geometry of C48 cation is a pure graphene carbon flakes (Lifshitz 2000; Zhen et al. 2014; Castel-lanos et al. 2018; Yang et al. 2021), as presented in Fig. 1. Based on the molecular structure of 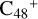 , we find that it has 20 outside edged-carbon sites and five typical carbon sites that can be used for the adduct reaction sites. Moreover, these five typical carbon sites can be treated as two types of carbon site: the first type is Duo-type,
, we find that it has 20 outside edged-carbon sites and five typical carbon sites that can be used for the adduct reaction sites. Moreover, these five typical carbon sites can be treated as two types of carbon site: the first type is Duo-type, 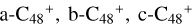 , and
, and 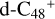 , C is located in its Duo carbon sites; the second type is Solo-type,
, C is located in its Duo carbon sites; the second type is Solo-type, 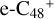 , C is located in its Solo carbon sites. As symmetrical carbon sites, we also labeled the other two carbon sites as a′ and e′. Furthermore, we will not consider the other 28 inside-carbon sites for the adduct reaction.
, C is located in its Solo carbon sites. As symmetrical carbon sites, we also labeled the other two carbon sites as a′ and e′. Furthermore, we will not consider the other 28 inside-carbon sites for the adduct reaction.
In addition, as presented in Fig. 1, we also give the C-C-C angle of C48 cation: ∡α = a′Ca = 136.35°, ∡β = bCc = 136.55°, ∡γ = dCe = 131.06°, and ∡δ = eCC = e′CC = 122.13°. We can see the angle of ∡δ = eCC = e′CC is smaller than ∡α = a′Ca, namely, there is a difference of 14.22°. The smaller C-C-C angel will facilitate some adduct reactions, such as intermolecular hydrogen transfer (Castellanos et al. 2018; Zhang et al. 2019).
The chemical reactivity of organic molecules strongly depends on their functional structures and their changes during chemical or biological processes (Engel & Macko 1997; Snyder 1997). Since our study assumes that pyroglutaminol molecules are all isolated in the gas phase, we mainly take pyroglutaminol (pgn, C5H9NO2) in its neutral form, and we assume that pyroglu-taminol maintain their structure during the vaporization process. Organic molecules have many different functional groups; the functional group of the organic molecule may play a decisive role in its chemical reactivity. For pyroglutaminol, four typical functional parts are labeled by bubbles. Each bubble represents one type of functional group: secondary amine (>NH), ketone (>C=O), methylene (>CH2), and primary alcohol (-CH2OH), respectively, as presented in Fig. 1.
In general, the covalently bonded clusters are more stable than the Van der Waals bonded clusters. Also, the Van der Waals bonded clusters are usually through an three-dimensional adduct reaction pathway, and the covalently bonded clusters are through an two-dimensional adduct reaction pathway, so we give priority to the consideration of an adduct reaction pathway through the quasi-two-dimensional one before considering it on the basis of the three-dimensional one. To our current work, pgn and C48+ are both quasi-two-dimensional planar molecules. Since the actual situation is very complicated, we also give priority to the consideration of covalently bonded clusters before their Van der Waals bonded clusters, namely, considering the adduct reaction pathway through the quasi-two-dimensional one before considering it through the three-dimensional one.
2.2 Reaction pathways and the optimized structures of  (1)
(1)
In Figs. 2 and 3, the reaction pathways and the optimized structures of 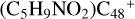 are presented. Pyroglutaminol molecules have four active functional groups, so four types of formation reaction pathway are considered for the newly formed clusters, as presented in Fig. 2A (with >NH), Fig. 3A (with >C=O), Fig. 3B (with -CH2OH), and Fig. 3C (with >CH2), respectively.
are presented. Pyroglutaminol molecules have four active functional groups, so four types of formation reaction pathway are considered for the newly formed clusters, as presented in Fig. 2A (with >NH), Fig. 3A (with >C=O), Fig. 3B (with -CH2OH), and Fig. 3C (with >CH2), respectively.
In Fig. 2A, six possible reaction pathways and the optimized structures of 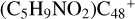 are obtained based on the >NH functional groups of pyroglutaminol and five typical edged-carbon sites of
are obtained based on the >NH functional groups of pyroglutaminol and five typical edged-carbon sites of 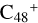 . To
. To 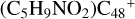 (a-P1, b-P1, c-P1, d-P1, and e-P1), the >NH functional groups of pyroglu-taminol and
(a-P1, b-P1, c-P1, d-P1, and e-P1), the >NH functional groups of pyroglu-taminol and 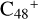 (a, b, c, d, and e) is combined via one C-N single bond, C is from
(a, b, c, d, and e) is combined via one C-N single bond, C is from 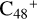 and N is from the >NH functional group. When forming a C-N single bond, the five-member ring in the pyroglutaminol is opened by disconnecting the CN bond. For
and N is from the >NH functional group. When forming a C-N single bond, the five-member ring in the pyroglutaminol is opened by disconnecting the CN bond. For 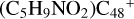 (e-P2), the >NH functional group and
(e-P2), the >NH functional group and 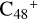 (e) is combined via one C-N single bond, C coming from
(e) is combined via one C-N single bond, C coming from 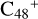 and N from the >NH functional group. Differently from
and N from the >NH functional group. Differently from 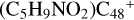 (e-P1), one hydrogen atom is directly transferred from the >NH unit to the graphene carbon (e′) and forms a new C-H unit, which leads to the 1, 2-addition product. Moreover, when forming a C-N single bond and hydrogen atom transfer, the five-member ring in the pyroglutaminol is still closed without opening.
(e-P1), one hydrogen atom is directly transferred from the >NH unit to the graphene carbon (e′) and forms a new C-H unit, which leads to the 1, 2-addition product. Moreover, when forming a C-N single bond and hydrogen atom transfer, the five-member ring in the pyroglutaminol is still closed without opening.
Clearly, as shown in Fig. 2A, all the reaction pathways are exothermic, with 1.4 eV for 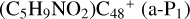 , with 1.4 eV for
, with 1.4 eV for 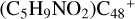 (b-Pı), with 1.4 eV for
(b-Pı), with 1.4 eV for 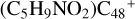 (c-P1), with 1.5 eV for (d-P1), with 2.8 eV for
(c-P1), with 1.5 eV for (d-P1), with 2.8 eV for 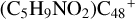 (e-P1), and with 5.0 eV for
(e-P1), and with 5.0 eV for 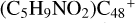 (e-P2). We can see that
(e-P2). We can see that 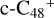 , and d-
, and d- 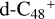 have a lower reactivity when compared to
have a lower reactivity when compared to 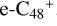 . The higher reactivity of
. The higher reactivity of 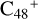 (e) is mainly due to the smaller angle of ∡δ = eCC = e′CC = 122.13°, and the smaller ∡δ = eCC = e′CC angel facilitate the hydrogen transfer. The formation behavior of
(e) is mainly due to the smaller angle of ∡δ = eCC = e′CC = 122.13°, and the smaller ∡δ = eCC = e′CC angel facilitate the hydrogen transfer. The formation behavior of 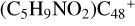 (a/b/c/d/e-P1) is very similar, and the formation pathway of
(a/b/c/d/e-P1) is very similar, and the formation pathway of 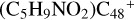 (e-P1) and
(e-P1) and 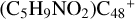 (e-P2) is energetically favored when compared to
(e-P2) is energetically favored when compared to 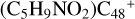 (a/b/c/d-Pı).
(a/b/c/d-Pı).
To understand the different formation behaviors of 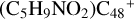 (e-P1) and
(e-P1) and 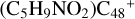 (e-P2), we consider the intermediate reaction states of
(e-P2), we consider the intermediate reaction states of 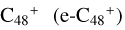 reaction with C5H9NO2 (>NH), presented in Fig. 2B. Along the lower red line reaction pathway, H from the >NH unit will gradually approach the reaction sites e′, as shown in the two intermediate states of [b1] and [b2]; this process is accompanied by reducing the binding energy of cluster cations. However, along the upper blue line reaction pathway, as shown in the two intermediate states of [a1] and [a2], a new C-N bond is formed, which connects C5H9NO2 and
reaction with C5H9NO2 (>NH), presented in Fig. 2B. Along the lower red line reaction pathway, H from the >NH unit will gradually approach the reaction sites e′, as shown in the two intermediate states of [b1] and [b2]; this process is accompanied by reducing the binding energy of cluster cations. However, along the upper blue line reaction pathway, as shown in the two intermediate states of [a1] and [a2], a new C-N bond is formed, which connects C5H9NO2 and 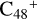 . In contrast, the O-C-N bond on the five-membered ring in C5H9NO2 is broken and does not maintain the five-membered ring configuration, H of the >NH unit was not involved in the reaction. The main reason is that C5H9NO2 molecule is an asymmetric planar ring structure. So, when the reaction occurs, the difference in the angle of the two molecular planes causes the difference in the reaction pattern; this is especially true for the hydrogen atom transfer reactions, which are more demanding in terms of their molecule structure conformation.
. In contrast, the O-C-N bond on the five-membered ring in C5H9NO2 is broken and does not maintain the five-membered ring configuration, H of the >NH unit was not involved in the reaction. The main reason is that C5H9NO2 molecule is an asymmetric planar ring structure. So, when the reaction occurs, the difference in the angle of the two molecular planes causes the difference in the reaction pattern; this is especially true for the hydrogen atom transfer reactions, which are more demanding in terms of their molecule structure conformation.
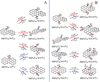 |
Fig. 4 Panel A: reaction pathways and optimized structures of |
2.3 Reaction pathways and the optimized structures of 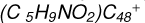 (2)
(2)
In Fig. 3A, two possible reaction pathways and the optimized structures of 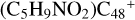 are obtained based on the >C=O functional group, and two typical edged-carbon sites of
are obtained based on the >C=O functional group, and two typical edged-carbon sites of 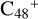 (a, and e) are considered. With regard to
(a, and e) are considered. With regard to 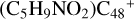 (a-P2, e-P3), the >C=O functional group and
(a-P2, e-P3), the >C=O functional group and 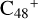 (a, and e) are combined via one C-O single bond, C is from
(a, and e) are combined via one C-O single bond, C is from 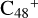 and O is from the >C=O functional group. The five-member ring in the pyroglutaminol is still closed without opening. Clearly, all the reaction are exothermic, with 1.5 eV for
and O is from the >C=O functional group. The five-member ring in the pyroglutaminol is still closed without opening. Clearly, all the reaction are exothermic, with 1.5 eV for 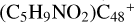 (a-P2) and with 2.8 eV for
(a-P2) and with 2.8 eV for 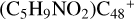 (e-P3). Similarly, with >C=O functional groups, we can see
(e-P3). Similarly, with >C=O functional groups, we can see 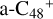 has a lower reactivity when compare to
has a lower reactivity when compare to 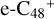 , the formation pathway of
, the formation pathway of 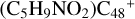 (e-P3) is energetically favored when compared to
(e-P3) is energetically favored when compared to 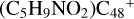 (a-P2).
(a-P2).
In Fig. 3B, four possible reaction pathways and the optimized structures of 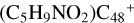 are obtained based on the -CH2OH functional group and two typical edged-carbon sites of
are obtained based on the -CH2OH functional group and two typical edged-carbon sites of 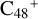 (a, and e). When the -CH2OH functional group reaction with
(a, and e). When the -CH2OH functional group reaction with 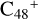 (a) takes place, two possible cluster isomers are formed: to
(a) takes place, two possible cluster isomers are formed: to  (a-P3), the -CH2OH functional group and
(a-P3), the -CH2OH functional group and 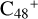 (a) are combined via one C-O single bond, C is from
(a) are combined via one C-O single bond, C is from 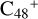 and O is from the -CH2OH functional group; with regard to
and O is from the -CH2OH functional group; with regard to 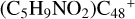 (a-P4), which is a Van der Waals cluster cations, the -CH2OH functional group will transfer to
(a-P4), which is a Van der Waals cluster cations, the -CH2OH functional group will transfer to 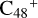 (a), and form a new C-C bond, while the rest of molecule (C4H6NO) will be bonded with [C48-CH2OH]+ through the Van der Waals bond.
(a), and form a new C-C bond, while the rest of molecule (C4H6NO) will be bonded with [C48-CH2OH]+ through the Van der Waals bond.
When the -CH2OH functional group reaction with 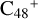 (e), two possible cluster isomers are also formed: to
(e), two possible cluster isomers are also formed: to 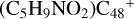 (e-P4), the -CH2OH functional group and
(e-P4), the -CH2OH functional group and 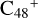 (e) are combined via one C-O single bond, and C is from
(e) are combined via one C-O single bond, and C is from 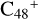 and O is from the CH2OH functional group; with regard to
and O is from the CH2OH functional group; with regard to 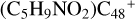 (e-P5), the CH2OH functional group and
(e-P5), the CH2OH functional group and 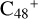 (e) are combined via one C-O single bond, and C is from
(e) are combined via one C-O single bond, and C is from 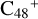 and O is from the -CH2OH functional group, and one hydrogen atom is directly transferred from -CH2OH unit to the graphene carbon (e′) to form a new C-H unit, which leads to the 1,2-addition product. The five-member ring in the pyroglutaminol is still closed without opening to these reaction pathways.
and O is from the -CH2OH functional group, and one hydrogen atom is directly transferred from -CH2OH unit to the graphene carbon (e′) to form a new C-H unit, which leads to the 1,2-addition product. The five-member ring in the pyroglutaminol is still closed without opening to these reaction pathways.
All the reaction are exothermic, with 0.4 eV for 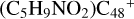 (a-P3), with 1.6 eV for
(a-P3), with 1.6 eV for 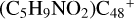 (a-P4), with 1.7 eV for
(a-P4), with 1.7 eV for 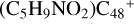 (e-P4), with 4.8 eV for
(e-P4), with 4.8 eV for 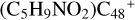 (e-P5). Similarly, with CH2OH functional groups, we can see
(e-P5). Similarly, with CH2OH functional groups, we can see 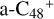 have a lower reactivity when compared to
have a lower reactivity when compared to 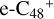 , the formation pathway of
, the formation pathway of 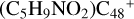 (e-P5) is energetically favored when compared to
(e-P5) is energetically favored when compared to 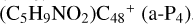 .
.
In Fig. 3C, two possible reaction pathways and the optimized structures of 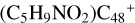 are obtained based on the >CH2 functional group, and two typical edged-carbon sites of
are obtained based on the >CH2 functional group, and two typical edged-carbon sites of 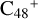 (a, and e) are selected. With regard to
(a, and e) are selected. With regard to 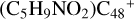 (a-P5) and
(a-P5) and 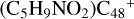 (e-P6), the >CH2 functional group and
(e-P6), the >CH2 functional group and 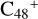 (a) or
(a) or 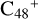 (e) is combined via one C-C single bond, one C is from
(e) is combined via one C-C single bond, one C is from 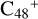 and the other C is from the >CH2 functional group, one hydrogen atom is directly transferred from >NH unit to the graphene carbon
and the other C is from the >CH2 functional group, one hydrogen atom is directly transferred from >NH unit to the graphene carbon 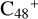 (a′, or e′) and form a C-H unit; in the meantime, a C-C single bond and hydrogen atom are transferred, while the five-member ring in the pyroglutaminol is opened by disconnecting the C-C bond. Clearly, all the reaction are exothermic, with 2.6 eV for
(a′, or e′) and form a C-H unit; in the meantime, a C-C single bond and hydrogen atom are transferred, while the five-member ring in the pyroglutaminol is opened by disconnecting the C-C bond. Clearly, all the reaction are exothermic, with 2.6 eV for 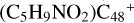 (a-P5), with 4.7 eV for
(a-P5), with 4.7 eV for 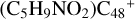 (e-P6). Similarly, with >CH2 functional groups, we can see a-
(e-P6). Similarly, with >CH2 functional groups, we can see a- have a lower reactivity when compare to e-
have a lower reactivity when compare to e-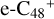 , the formation pathway of
, the formation pathway of 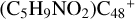 (e-P6) is energetically favored when compared to
(e-P6) is energetically favored when compared to 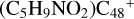 (a-P5).
(a-P5).
As we can see, the exothermic energy is relatively higher that can stabilize the whole clusters, so we propose that 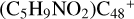 formed in the lab is a mixture of clusters with all different possible isomers. In the process of adding pyroglutaminol on the
formed in the lab is a mixture of clusters with all different possible isomers. In the process of adding pyroglutaminol on the 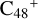 edges, the binding energy and the bond type are different, namely, all the polymerization reactions primarily depend on the location of the effective carbon edges. The solo site carbons, e-
edges, the binding energy and the bond type are different, namely, all the polymerization reactions primarily depend on the location of the effective carbon edges. The solo site carbons, e-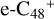 , have a higher reactivity than the duo site carbons of a/b/c/d-
, have a higher reactivity than the duo site carbons of a/b/c/d-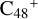 when reacting with pyroglutaminol. In addition, we also conclude that the H migration plays an important role in the adduction process, and the barrier for H migration is relatively lower, which can happen in the adduction process. However, with H immigration, PAH species will have fewer available carbon sites for future adduct reactions.
when reacting with pyroglutaminol. In addition, we also conclude that the H migration plays an important role in the adduction process, and the barrier for H migration is relatively lower, which can happen in the adduction process. However, with H immigration, PAH species will have fewer available carbon sites for future adduct reactions.
 |
Fig. 5 Panel A: reaction pathways and optimized structures of |
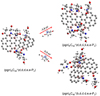 |
Fig. 6 Reaction pathways and the optimized structures of |
2.4 Reaction pathways and the optimized structures of 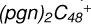
Based on the obtained calculation results of 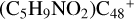 in Figs. 2 and 3, we can see the >NH functional groups of pyroglutaminol have a relative higher reactivity when compared to the other functional group. So for simplicity, to the subsequent adduct reaction pathways, from
in Figs. 2 and 3, we can see the >NH functional groups of pyroglutaminol have a relative higher reactivity when compared to the other functional group. So for simplicity, to the subsequent adduct reaction pathways, from 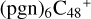 , we only calculated the >NH functional group as an example for the reaction pathways. As to the configuration of
, we only calculated the >NH functional group as an example for the reaction pathways. As to the configuration of 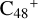 , we only consider five reaction sites (a, b, c, d, and e). The reaction sites in symmetrical position have the same reaction activity, for example, we labeled the other two carbon sites as
, we only consider five reaction sites (a, b, c, d, and e). The reaction sites in symmetrical position have the same reaction activity, for example, we labeled the other two carbon sites as 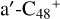 have the same reaction activity as the carbon sites a-
have the same reaction activity as the carbon sites a-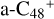 , and
, and 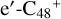 ave the same reaction activity as the carbon sites e-
ave the same reaction activity as the carbon sites e-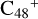 . According to the formation behavior of
. According to the formation behavior of 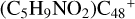 , we believe that the reaction site that is symmetrical to e-
, we believe that the reaction site that is symmetrical to e-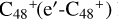 has a priority react possibility.
has a priority react possibility.
In Fig. 4A, the reaction pathways and the optimized structures of 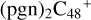 are illustrated. We mainly focus on two types of reaction pathway:
are illustrated. We mainly focus on two types of reaction pathway: 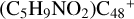 (e-P2, in red path), and
(e-P2, in red path), and 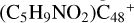 (e-P1, in blue path), respectively.
(e-P1, in blue path), respectively.
To the red path, it shows the reaction pathways for the different carbon react sites of 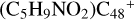 (e-P2) (a, and e) reacting with C5H9NO2 (>NH), leading to the formation of
(e-P2) (a, and e) reacting with C5H9NO2 (>NH), leading to the formation of 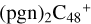 (e,e-P2),
(e,e-P2), 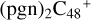 (a,e-P2), and
(a,e-P2), and 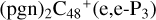 . With regard to
. With regard to 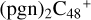 (e,e-P2), the >NH functional group and
(e,e-P2), the >NH functional group and  (e-P2) (e) are combined via one C-N single bond, and C is from
(e-P2) (e) are combined via one C-N single bond, and C is from 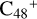 and N is from the >NH functional group. One hydrogen atom is directly transferred from >NH unit to the graphene carbon (e′) and form a C-H unit, which leads to the 1, 2-addition product, and in the forming of C-N single bond and hydrogen atom transfer, the five member ring in the pyroglutaminol is still closed without opening. To
and N is from the >NH functional group. One hydrogen atom is directly transferred from >NH unit to the graphene carbon (e′) and form a C-H unit, which leads to the 1, 2-addition product, and in the forming of C-N single bond and hydrogen atom transfer, the five member ring in the pyroglutaminol is still closed without opening. To 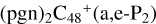 and
and 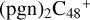 (e,e-P3), the >NH functional group and
(e,e-P3), the >NH functional group and 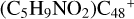 (e-P2) (a, and e) is combined via one C-N single bond, C is from
(e-P2) (a, and e) is combined via one C-N single bond, C is from 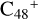 and N is from the >NH functional group, in the meantime of forming C-N single bond, the five member ring in the pyroglutaminol is opened by disconnecting the CN bond.
and N is from the >NH functional group, in the meantime of forming C-N single bond, the five member ring in the pyroglutaminol is opened by disconnecting the CN bond.
To the blue path, it shows the reaction pathways for 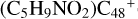 (e-P1) (e) reacting with C5H9NO2 (>NH), leading to the formation of
(e-P1) (e) reacting with C5H9NO2 (>NH), leading to the formation of 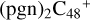 (e,e-P3), and
(e,e-P3), and 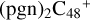 (e,e-P1). To
(e,e-P1). To 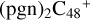 (e,e-P3), the >NH functional group and
(e,e-P3), the >NH functional group and 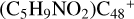 (e-P1) (e) are combined via one C-N single bond, C is from
(e-P1) (e) are combined via one C-N single bond, C is from 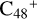 and N is from the >NH functional group. One hydrogen atom is directly transferred from the >NH unit to the graphene carbon (e′) and form a C-H unit, which leads to the 1, 2-addition product, and in the forming of C-N single bond and hydrogen atom transfer, the five-member ring in the pyroglutaminol is still closed without opening. With regard to
and N is from the >NH functional group. One hydrogen atom is directly transferred from the >NH unit to the graphene carbon (e′) and form a C-H unit, which leads to the 1, 2-addition product, and in the forming of C-N single bond and hydrogen atom transfer, the five-member ring in the pyroglutaminol is still closed without opening. With regard to 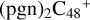 (e,e-P1), the >NH functional group and
(e,e-P1), the >NH functional group and 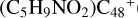 (e-P1) (e) are combined via one C-N single bond, and C is from
(e-P1) (e) are combined via one C-N single bond, and C is from 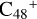 and N is from the >NH functional group, in the meantime the C-N single bond is formed, while the five-member ring in the pyroglutaminol is opened by disconnecting the CN bond. The carbon atoms of carbonyl groups are connected to
and N is from the >NH functional group, in the meantime the C-N single bond is formed, while the five-member ring in the pyroglutaminol is opened by disconnecting the CN bond. The carbon atoms of carbonyl groups are connected to 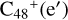 , with a new seven-membered ring appearing.
, with a new seven-membered ring appearing.
As we can see in Fig. 4A, all the reaction are exothermic, with 5.0 eV for 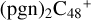 (e,e-P2), 1.2 eV for
(e,e-P2), 1.2 eV for 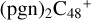 (a,e-P2), 2.7 eV for
(a,e-P2), 2.7 eV for 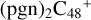 (e,e-P3) from
(e,e-P3) from 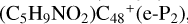 , (e-P2) 4.9 eV for
, (e-P2) 4.9 eV for 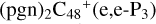 (e,e-P3) from
(e,e-P3) from 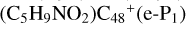 , and 4.2 eV for
, and 4.2 eV for 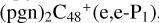 , respectively. Similarly, we can see
, respectively. Similarly, we can see 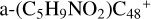 has a lower reactivity when compared to
has a lower reactivity when compared to 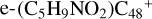 , and the formation pathway of
, and the formation pathway of 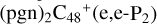 is energetically favored when compared to
is energetically favored when compared to 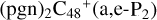 . We also noted that: with regard to
. We also noted that: with regard to 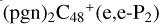 , four e type of carbon sites are already involved in the reaction; to
, four e type of carbon sites are already involved in the reaction; to 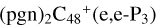 , three e type of carbon sites are involved in the reaction; to
, three e type of carbon sites are involved in the reaction; to 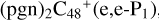 , three e type of carbon sites are involved in the reaction.
, three e type of carbon sites are involved in the reaction.
 |
Fig. 7 Expanded tree (in building block pathways) shows the trunk and branches of these various formation pathways for these clusters, |
2.5 Reaction pathways and the optimized structures of 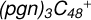
In Fig. 4B, the reaction pathways and the optimized structures of 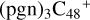 are illustrated. From the calculation results presented above, the “a” type of carbon site has a lower reactivity when compared to the “e” type of the carbon site. Hence, the “e” type of carbon site has a higher possibility to engage in a adduction reaction. If there are still “e” type carbon sites left, then we would choose the “e” type of carbon sites first; after that, we choose a or d type of carbon sites for the next level of reaction adduction.
are illustrated. From the calculation results presented above, the “a” type of carbon site has a lower reactivity when compared to the “e” type of the carbon site. Hence, the “e” type of carbon site has a higher possibility to engage in a adduction reaction. If there are still “e” type carbon sites left, then we would choose the “e” type of carbon sites first; after that, we choose a or d type of carbon sites for the next level of reaction adduction.
There is two e type of carbon sites left for the react adduction on 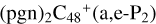 , then we chose e carbon sites, for the formation pathway of
, then we chose e carbon sites, for the formation pathway of 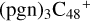 . There is no e type of carbon sites for the react adduction on
. There is no e type of carbon sites for the react adduction on 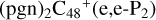 , then we chose a and d carbon sites, for the formation pathway of
, then we chose a and d carbon sites, for the formation pathway of 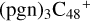 . For simplicity, to
. For simplicity, to 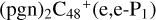 , and
, and 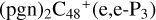 , even when there is one “e” type of carbon site left, we would still choose the “d” type of carbon sites for the adduct reaction.
, even when there is one “e” type of carbon site left, we would still choose the “d” type of carbon sites for the adduct reaction.
To the red path, it shows the react pathways that the different active sites of 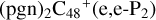 (a, and d) react with C5H9NO2 (>NH), to the formation of
(a, and d) react with C5H9NO2 (>NH), to the formation of 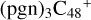 (d,e,e-P2), and
(d,e,e-P2), and 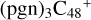 (a,e,e-P2) (a,e,e-P2).
(a,e,e-P2) (a,e,e-P2). 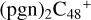 (a,e,e-P2) (a,e-P2) (e) react with C5H9NO2 (>NH), to the formation of
(a,e,e-P2) (a,e-P2) (e) react with C5H9NO2 (>NH), to the formation of 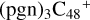 (a,e,e-P2) (a,e,e-P2).
(a,e,e-P2) (a,e,e-P2). 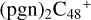 (a,e,e-P2) (e,e-P3) (d) react with C5H9NO2 (>NH), to the formation of
(a,e,e-P2) (e,e-P3) (d) react with C5H9NO2 (>NH), to the formation of 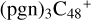 (a,e,e-P2) (d,e,e-P3).
(a,e,e-P2) (d,e,e-P3).
To 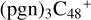 (a,e,e-P2) (a,e,e-P2) and
(a,e,e-P2) (a,e,e-P2) and 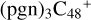 (a,e,e-P2) (d,e,e-P2), the >NH functional group and
(a,e,e-P2) (d,e,e-P2), the >NH functional group and 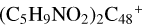 (e,e-P2) (a, and d) are combined via one C-N single bond, C is from
(e,e-P2) (a, and d) are combined via one C-N single bond, C is from 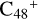 and N is from the >NH functional group, in the meantime, while the C-N single bond is formed, the five-member ring in the pyroglutaminol is opened by disconnecting the CN bond. To the formation pathway of
and N is from the >NH functional group, in the meantime, while the C-N single bond is formed, the five-member ring in the pyroglutaminol is opened by disconnecting the CN bond. To the formation pathway of 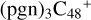 (a,e,e-P2), the >NH functional group and
(a,e,e-P2), the >NH functional group and 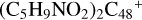 (a,e-P2) (e) are combined via one C-N single bond, and C is from
(a,e-P2) (e) are combined via one C-N single bond, and C is from 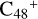 and N is from the >NH functional group. One hydrogen atom is directly transferred from the >NH unit to the graphene carbon (e′) and forms a C-H unit, which leads to the 1, 2-addition product. In the forming C-N single bond and hydrogen atom transfer, the five-member ring in the pyroglutaminol is closed without opening. Interestingly, the carbon atoms of carbonyl groups are connected to b-
and N is from the >NH functional group. One hydrogen atom is directly transferred from the >NH unit to the graphene carbon (e′) and forms a C-H unit, which leads to the 1, 2-addition product. In the forming C-N single bond and hydrogen atom transfer, the five-member ring in the pyroglutaminol is closed without opening. Interestingly, the carbon atoms of carbonyl groups are connected to b-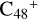 , with a new six-membered ring appearing. To
, with a new six-membered ring appearing. To 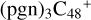 (d,e,e-P3), the >NH functional group and
(d,e,e-P3), the >NH functional group and 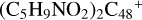 (d) is combined via one C-N single bond, and C is from
(d) is combined via one C-N single bond, and C is from 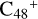 and N is from the >NH functional group, in the meantime, a C-N single bond is formed and the five-member ring in the pyroglutaminol is opened by disconnecting the CN bond.
and N is from the >NH functional group, in the meantime, a C-N single bond is formed and the five-member ring in the pyroglutaminol is opened by disconnecting the CN bond.
With regard to the blue path, it shows the reaction pathways that 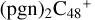 (e,e-P1) (d) react with C5H9NO2 (>NH), leading to the formation of
(e,e-P1) (d) react with C5H9NO2 (>NH), leading to the formation of 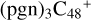 (d,e,e-P1). With regard to the formation pathway of
(d,e,e-P1). With regard to the formation pathway of 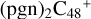 (d,e,e-P1), the >NH functional group and
(d,e,e-P1), the >NH functional group and 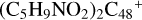 (d) are combined via one C-N single bond, and C is from
(d) are combined via one C-N single bond, and C is from 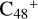 and N is from the >NH functional group. The five member ring in the pyroglutaminol is opened by disconnecting the CN bond, and the carbon atoms of carbonyl groups are connected to
and N is from the >NH functional group. The five member ring in the pyroglutaminol is opened by disconnecting the CN bond, and the carbon atoms of carbonyl groups are connected to 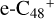 , and a new eight membered ring appears.
, and a new eight membered ring appears.
As we can see in Fig. 4B, all the reaction are exothermic, with 1.5 eV for 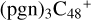 (d,e,e-P2), 1.8 eV (the reactants is
(d,e,e-P2), 1.8 eV (the reactants is 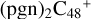 (e,e-P2)) and 5.4 eV (the reactants is
(e,e-P2)) and 5.4 eV (the reactants is 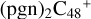 (a,e-P2)) for
(a,e-P2)) for 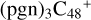 (a,e,e-P2), 3.4 eV for
(a,e,e-P2), 3.4 eV for 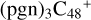 (d,e,e-P3), and 1.7 eV for
(d,e,e-P3), and 1.7 eV for 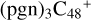 (d,e,e-P1), respectively.
(d,e,e-P1), respectively.
As we can see from the calculation results presented above, the reaction pathways and the optimized structures of 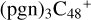 is very complex. We note that we only calculated and presented the initial step of the adduct reaction at the beginning of the ion-molecule collision reaction process. In further reaction adduction, the whole molecular structure will adjust, with some intra-molecular isomerization process, the other functional group of pyroglutaminol may also add to the carbon sites of
is very complex. We note that we only calculated and presented the initial step of the adduct reaction at the beginning of the ion-molecule collision reaction process. In further reaction adduction, the whole molecular structure will adjust, with some intra-molecular isomerization process, the other functional group of pyroglutaminol may also add to the carbon sites of 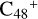 . Based on that, for the reaction pathways and the optimized structures of
. Based on that, for the reaction pathways and the optimized structures of 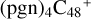 ,
, 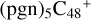 and
and 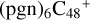 , we just briefly describe the formation behaviors. In addition, we note that these molecular clusters are very large, so we chose a relatively smaller basis set for the calculation: from 6-311++G** basis setto 6-31+G**.
, we just briefly describe the formation behaviors. In addition, we note that these molecular clusters are very large, so we chose a relatively smaller basis set for the calculation: from 6-311++G** basis setto 6-31+G**.
2.6 Reaction pathways and the optimized structures of 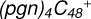
In Fig. 5A, the reaction pathways and the optimized structures of 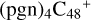 are illustrated.
are illustrated.
With regard to the red path, it shows the react pathways that the different active sites of 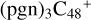 (d,e,e-P2) (a, and d) react with
(d,e,e-P2) (a, and d) react with 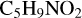 (>NH), to the formation of
(>NH), to the formation of 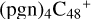 (a,d,e,e-P2),
(a,d,e,e-P2), 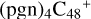 (d,d,e,e-P2), and
(d,d,e,e-P2), and 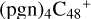 (d,d,e,e-P3).
(d,d,e,e-P3).
With regard to 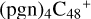 (a,d,e,e-P2), the formation pathway is very complex when C5H9NO2 (>NH) react with
(a,d,e,e-P2), the formation pathway is very complex when C5H9NO2 (>NH) react with 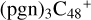 (d,e,e-P2) (a), the five-membered ring in C5H9NO2 is broken, meanwhile, the carbon atoms of the carbonyl groups are connected to one b carbon site of
(d,e,e-P2) (a), the five-membered ring in C5H9NO2 is broken, meanwhile, the carbon atoms of the carbonyl groups are connected to one b carbon site of 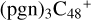 , and a new seven-membered ring appears. When C5H9NO2 (>NH) react with
, and a new seven-membered ring appears. When C5H9NO2 (>NH) react with 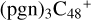 (d,e,e-P2) (d), two similar isomer of
(d,e,e-P2) (d), two similar isomer of 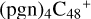 (d,d,e,e-P2) and
(d,d,e,e-P2) and 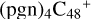 (d,d,e,e-P3) are formed. To the formation pathway of
(d,d,e,e-P3) are formed. To the formation pathway of 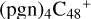 (d,d,e,e-P2), H atom from >NH is transferred to one b carbon site of
(d,d,e,e-P2), H atom from >NH is transferred to one b carbon site of 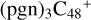 (d,e,e-P2), and instead of forming C-N bond but forming one C-O bond. During the bonding process of C-O, the intra-molecular structure undergoes a significant change. With regard to the formation pathway of
(d,e,e-P2), and instead of forming C-N bond but forming one C-O bond. During the bonding process of C-O, the intra-molecular structure undergoes a significant change. With regard to the formation pathway of 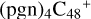 (d,d,e,e-P3), one C-N single bond is formed, C is from
(d,d,e,e-P3), one C-N single bond is formed, C is from 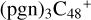 (d,e,e-P2) (d) and N is from the >NH functional group. In the meantime, the five-membered ring in C5H9NO2 is open, the carbon atoms of carbonyl groups are connected to
(d,e,e-P2) (d) and N is from the >NH functional group. In the meantime, the five-membered ring in C5H9NO2 is open, the carbon atoms of carbonyl groups are connected to 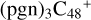 (d,e,e-P2) (c), and the oxygen atom of carbonyl group is connected to
(d,e,e-P2) (c), and the oxygen atom of carbonyl group is connected to 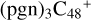 (d,e,e-P2) (d), and a new seven-membered ring and a five-membered ring appears.
(d,e,e-P2) (d), and a new seven-membered ring and a five-membered ring appears.
With regard to the blue path, it shows the react pathways that the different active sites of 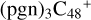 (d,e,e-P1) (a, and d) react with C5H9NO2 (>NH), to the formation of
(d,e,e-P1) (a, and d) react with C5H9NO2 (>NH), to the formation of 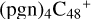 (a,d,e,e-P1), and
(a,d,e,e-P1), and 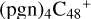 (d,d,e,e-P1). With regard to the formation pathway of
(d,d,e,e-P1). With regard to the formation pathway of 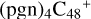 (d,d,e,e-P1), one C-N single bond is formed, C is from
(d,d,e,e-P1), one C-N single bond is formed, C is from 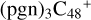 (d,e,e-P2) (d) and N is from the >NH functional group. H atom from >NH is transferred to one c carbon site of
(d,e,e-P2) (d) and N is from the >NH functional group. H atom from >NH is transferred to one c carbon site of 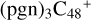 (d,e,e-P1), and form one new C-H bond. To
(d,e,e-P1), and form one new C-H bond. To 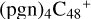 (a,d,e,e-P2), one C-N single bond is formed, C is from
(a,d,e,e-P2), one C-N single bond is formed, C is from 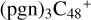 (d,e,e-P2) (a) and N is from the >NH functional group. In the meantime of forming C-N single bond, the five-member ring in the pyroglutaminol is opened by disconnecting the CN bond.
(d,e,e-P2) (a) and N is from the >NH functional group. In the meantime of forming C-N single bond, the five-member ring in the pyroglutaminol is opened by disconnecting the CN bond.
As we can see in Fig. 5A, all the reaction are exothermic, with 3.7 eV for 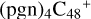 (a,d,e,e-P2), 3.2 eV for
(a,d,e,e-P2), 3.2 eV for 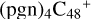 (d,d,e,e-P2) and 4.1 eV for
(d,d,e,e-P2) and 4.1 eV for 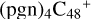 (d,d,e,e-P3), with 3.6 eV for
(d,d,e,e-P3), with 3.6 eV for 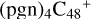 (d,d,e,e-P1), 1.5 eV for
(d,d,e,e-P1), 1.5 eV for 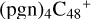 (a,d,e,e-P1), respectively.
(a,d,e,e-P1), respectively.
2.7 Reaction pathways and the optimized structures of 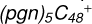
In Fig. 5B, the reaction pathways and the optimized structures of 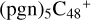 are illustrated.
are illustrated.
With regard to the red path, it shows the reaction pathway that the carbon sites of 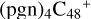 (d,d,e,e-P2) (d) react with C5H9NO2 (>NH), to the formation of
(d,d,e,e-P2) (d) react with C5H9NO2 (>NH), to the formation of 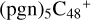 (d,d,d,e,e-P2), and
(d,d,d,e,e-P2), and 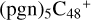 (d,d,d,e,e-P3); the carbon sites of
(d,d,d,e,e-P3); the carbon sites of 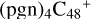 (a,d,e,e-P2) (a) react with C5H9NO2 (>NH), leading to the formation of
(a,d,e,e-P2) (a) react with C5H9NO2 (>NH), leading to the formation of 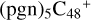 (a,a,d,e,e-P2).
(a,a,d,e,e-P2).
With regard to 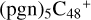 (d,d,d,e,e-P2), the formation pathway is very complex. One C-N single bond is formed, C is from
(d,d,d,e,e-P2), the formation pathway is very complex. One C-N single bond is formed, C is from 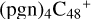 (d,d,e,e-P2) (d) and N is from the >NH functional group. In the meantime of forming C-N single bond, the five-member ring in the pyroglutaminol is opened by disconnecting the CN bond, the carbon atoms of carbonyl groups are connected to one c carbon site of
(d,d,e,e-P2) (d) and N is from the >NH functional group. In the meantime of forming C-N single bond, the five-member ring in the pyroglutaminol is opened by disconnecting the CN bond, the carbon atoms of carbonyl groups are connected to one c carbon site of 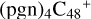 (d,d,e,e-P2), with a new seven-membered ring appears. As for
(d,d,e,e-P2), with a new seven-membered ring appears. As for 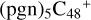 (d,d,d,e,e-P3), it is a Van der Waals cluster cations, which contains a hydrogen bond (O-H…O), with a bond length of 1.875 Å. To
(d,d,d,e,e-P3), it is a Van der Waals cluster cations, which contains a hydrogen bond (O-H…O), with a bond length of 1.875 Å. To 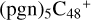 (a,a,d,e,e-P2), one C-N single bond is formed, C is from
(a,a,d,e,e-P2), one C-N single bond is formed, C is from 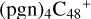 (a,d,e,e-P2) (a) and N is from the >NH functional group. In the meantime of forming C-N single bond, the five-member ring in the pyroglutaminol is opened by disconnecting the CN bond. This cluster contains 133 atoms with a diameter of 2.5 nm.
(a,d,e,e-P2) (a) and N is from the >NH functional group. In the meantime of forming C-N single bond, the five-member ring in the pyroglutaminol is opened by disconnecting the CN bond. This cluster contains 133 atoms with a diameter of 2.5 nm.
With regard to the blue path, it shows the reaction pathway in which 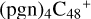 (d,d,e,e-P1) (d) reacts with C5H9NO2 (>NH), leading to the formation of
(d,d,e,e-P1) (d) reacts with C5H9NO2 (>NH), leading to the formation of 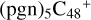 (d,d,d,e,e-P1). As for
(d,d,d,e,e-P1). As for 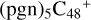 (d,d,d,e,e-P1), it is a Van der Waals cluster cation, which contains a hydrogen bond (O-H O), with a bond length of 2.007 Å.
(d,d,d,e,e-P1), it is a Van der Waals cluster cation, which contains a hydrogen bond (O-H O), with a bond length of 2.007 Å.
As we can see in Fig. 5B, all the reaction are exothermic, with 3.3 eV for 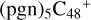 (d,d,d,e,e-P2), 1.2 eV for
(d,d,d,e,e-P2), 1.2 eV for 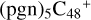 (d,d,d,e,e-P3), +1.0 eV for
(d,d,d,e,e-P3), +1.0 eV for 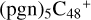 (a,a,d,e,e-P2), with 0.8 eV for
(a,a,d,e,e-P2), with 0.8 eV for 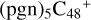 (d,d,d,e,e-P1), respectively.
(d,d,d,e,e-P1), respectively.
2.8 Reaction pathways and the optimized structures of 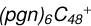
In Fig. 6, the reaction pathways and the optimized structures of 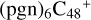 are illustrated. It shows the process that the different active sites of
are illustrated. It shows the process that the different active sites of 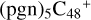 (d,d,d,e,e-P2) (b, and d) react with C5H9NO2 (>NH), to the formation of
(d,d,d,e,e-P2) (b, and d) react with C5H9NO2 (>NH), to the formation of 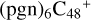 (d,d,d,d,e,e-P2), and
(d,d,d,d,e,e-P2), and 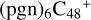 (b,d,d,d,e,e-P2). The cluster cation of
(b,d,d,d,e,e-P2). The cluster cation of 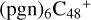 (d,d,d,d,e,e-P2) contains 150 atoms with a diameter of around 2.0 nm. As for
(d,d,d,d,e,e-P2) contains 150 atoms with a diameter of around 2.0 nm. As for 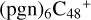 (b,d,d,d,e,e-P2), it is a Van der Waals cluster cation. All the reaction are exothermic, with 3.8 eV for
(b,d,d,d,e,e-P2), it is a Van der Waals cluster cation. All the reaction are exothermic, with 3.8 eV for 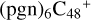 (d,d,d,d,e,e-P2), 0.9 eV for
(d,d,d,d,e,e-P2), 0.9 eV for 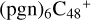 (b,d,d,d,e,e-P2).
(b,d,d,d,e,e-P2).
2.9 Discussion
Overall, the structure of newly formed species and the energy for these reaction pathways are obtained, and these theoretical calculation results are consistent with the experimental results obtained by (Hu et al. 2021a). The experimental results show thatPgn ccan form more complex PAH-organic molecular clusters (e.g., 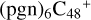 ). From the obtained calculation result, we can conclude: the ion-molecule reaction between
). From the obtained calculation result, we can conclude: the ion-molecule reaction between 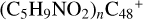 , n = [0,5]) and C5H9NO2 readily occur, resulting in a very large number of reaction pathways and very complex newly formed molecular clusters. These obtained molecules have a complex configuration, as well as an internal composition, and are large, which can be a good example and treated as the first formation step of prebiotic molecules in the interstellar environments (Herbst & van Dishoeck 2009; Jäger et al. 2009).
, n = [0,5]) and C5H9NO2 readily occur, resulting in a very large number of reaction pathways and very complex newly formed molecular clusters. These obtained molecules have a complex configuration, as well as an internal composition, and are large, which can be a good example and treated as the first formation step of prebiotic molecules in the interstellar environments (Herbst & van Dishoeck 2009; Jäger et al. 2009).
The exothermic energy for these reaction pathways is relatively higher that can stabilize the whole cluster, so we propose that 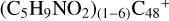 formed in the lab are mixture clusters with all different possible isomers. The solo site carbons,
formed in the lab are mixture clusters with all different possible isomers. The solo site carbons, 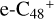 , have higher reactivity than the duo site carbons of a/b/c/d-
, have higher reactivity than the duo site carbons of a/b/c/d-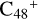 when reacting with pyroglutaminol. The >NH functional group has a relatively higher reactivity when compared to other functional groups (>C=O, CH2, and -CH2OH) of pyroglutaminol. For simplifying, >NH functional groups of pyroglutaminol are selected as an example of the reaction sites of pyroglutaminol for all the high levels of adduct reactions. In addition, we give our idea that the collision reaction transition state maybe exists but not possible very higher, the main evidence is from the obtained experimental result, the formation reaction between PAHs cations with neutral molecules in the gas phase are very efficient.
when reacting with pyroglutaminol. The >NH functional group has a relatively higher reactivity when compared to other functional groups (>C=O, CH2, and -CH2OH) of pyroglutaminol. For simplifying, >NH functional groups of pyroglutaminol are selected as an example of the reaction sites of pyroglutaminol for all the high levels of adduct reactions. In addition, we give our idea that the collision reaction transition state maybe exists but not possible very higher, the main evidence is from the obtained experimental result, the formation reaction between PAHs cations with neutral molecules in the gas phase are very efficient.
These clusters (e.g., graphene carbon cluster and organic molecules) provide a possible formation and chemical-evolution route for the large complex prebiotic compounds in bottom-up and energy allowed processes in the ISM. The calculation results show that it is feasible and possible for 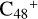 to connect with six pyroglutaminol molecules surrounding the
to connect with six pyroglutaminol molecules surrounding the 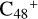 (in cova-lently bonded compounds or in Van der Waals bonded ones). The size of these
(in cova-lently bonded compounds or in Van der Waals bonded ones). The size of these 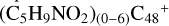 derived clusters approach that of very small grains. The nucleus-to-nucleus diameter of
derived clusters approach that of very small grains. The nucleus-to-nucleus diameter of 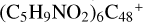 is measured in the range of 2–3 nm.
is measured in the range of 2–3 nm.
Figure 7 illustrates the building block mechanism for the formation of large prebiotic compounds; an expanded tree (in building block pathway) shows the trunk and branches of these various formation pathways for these clusters, 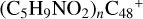 , n from 0 to 6 (based on the >NH functional groups of pyroglutaminol). Two trunks in solid red and solid blue lines with some branches in dotted line are presented. In addition, from the formation pathway of
, n from 0 to 6 (based on the >NH functional groups of pyroglutaminol). Two trunks in solid red and solid blue lines with some branches in dotted line are presented. In addition, from the formation pathway of 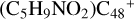 to
to 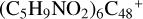 , we can see that the reactivity of these molecular clusters does not vary much, while the size of the clusters gets larger. The higher reactivity of graphene species may indicate that complex organic molecules or other related prebiotic compounds can efficiently accrete on small carbon dust grains in the ISM (Tielens 2013; Gavilan et al. 2020).
, we can see that the reactivity of these molecular clusters does not vary much, while the size of the clusters gets larger. The higher reactivity of graphene species may indicate that complex organic molecules or other related prebiotic compounds can efficiently accrete on small carbon dust grains in the ISM (Tielens 2013; Gavilan et al. 2020).
Supposing we set aside the limitations of our experimental conditions, we could make a relatively reasonable guess that such molecular clusters can continue to grow in their current size (possibly adduct on the previous adducted pyroglutaminol for the third layer of clusters). That is to say that in a favorable environment of interstellar space, it may be possible to eventually form molecular clusters on the order of micrometers through the ways mentioned above (Jäger et al. 2009; Berné et al. 2015). Interestingly, the later adducted pyroglutaminol might form an amide bond with the previously adducted organic molecules on the surface of clusters. These two interstellar species may form clusters with multi-shell structural features, and then this structure can connect with multi-pyroglutaminol bearing PAHs to form clusters in large size (Rapacioli et al. 2006; Candian et al. 2018).
3 IR spectrum of PAH-organic molecule clusters
In brief, PAH-organic molecule clusters (e.g., the garphene carbon cluster-organic molecule cluster cations, 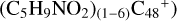 may serve as candidates of interest for the IR interstellar bands in motivating spectroscopic studies (Tielens 2013). In Fig. 8, computed vibrational normal modes of
may serve as candidates of interest for the IR interstellar bands in motivating spectroscopic studies (Tielens 2013). In Fig. 8, computed vibrational normal modes of  , C5H9NO2, and
, C5H9NO2, and 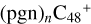 , n=[1,5]) are represented in the range of 0–4000 cm−1: panel A is the IR spectrum of
, n=[1,5]) are represented in the range of 0–4000 cm−1: panel A is the IR spectrum of 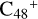 ; panel B is the IR spectrum of
; panel B is the IR spectrum of 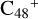 (e-P2); panel C is the IR spectrum of
(e-P2); panel C is the IR spectrum of  (e,e-P2); panel D is the IR spectrum of
(e,e-P2); panel D is the IR spectrum of 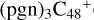 (d,2e-P2); panel E is the IR spectrum of
(d,2e-P2); panel E is the IR spectrum of 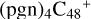 (2d,2e-P2); panel F is the IR spectrum of
(2d,2e-P2); panel F is the IR spectrum of 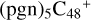 (3d,2e-P2); panel G is the IR spectrum of C5H9NO2. Here, we are using the 6-311++G** basis set. The vibrational band positions scaled by a uniform factor of 0.9670, and the black line is the simulated spectra by Gaussians with a full width half-maximum of 4 cm−1 (Boersma et al. 2014).
(3d,2e-P2); panel G is the IR spectrum of C5H9NO2. Here, we are using the 6-311++G** basis set. The vibrational band positions scaled by a uniform factor of 0.9670, and the black line is the simulated spectra by Gaussians with a full width half-maximum of 4 cm−1 (Boersma et al. 2014).
As shown in Fig. 8, the IR spectra of each individual cluster 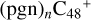 , n=[1,5] are very complex. We only conclude two characteristic peaks in the range of 1500–2500 cm−1: the stretch vibration of C=O and the stretch vibration of C=C. In Fig. 8A, with regard to the IR spectrum of
, n=[1,5] are very complex. We only conclude two characteristic peaks in the range of 1500–2500 cm−1: the stretch vibration of C=O and the stretch vibration of C=C. In Fig. 8A, with regard to the IR spectrum of 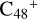 , the characteristic peak of the stretch vibration of C=C is 2000 cm−1 (these peripheral carbons); with regard to the IR spectrum of C5H9NO2, in Fig. 8G, the characteristic peak of the stretch vibration of C=O is 1809 cm−1. As shown in Figs. 8B-F, the spectra changes as the size of the cluster grows from (pgn)
, the characteristic peak of the stretch vibration of C=C is 2000 cm−1 (these peripheral carbons); with regard to the IR spectrum of C5H9NO2, in Fig. 8G, the characteristic peak of the stretch vibration of C=O is 1809 cm−1. As shown in Figs. 8B-F, the spectra changes as the size of the cluster grows from (pgn) 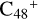 (e-P2) to
(e-P2) to 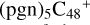 (3d,2e-P2): when these clusters are smaller, the spectra of these clusters tend to be more similar to the graphene carbon clusters
(3d,2e-P2): when these clusters are smaller, the spectra of these clusters tend to be more similar to the graphene carbon clusters 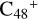 , and when these clusters are larger, the spectra of the clusters tend to be more similar to C5H9NO2. For example, to the IR spectrum of
, and when these clusters are larger, the spectra of the clusters tend to be more similar to C5H9NO2. For example, to the IR spectrum of 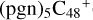 (3d,2e-P2), the intensity of the characteristic peaks (2000 cm−1) of the stretch vibration of C=C is decreasing and approaching zero, which agrees with almost all the peripheral carbons of the carbon skeleton of C48 being occupied; and the intensity of the characteristic peaks of the stretch vibration of C=O is increasing and approaching C5H9NO2.
(3d,2e-P2), the intensity of the characteristic peaks (2000 cm−1) of the stretch vibration of C=C is decreasing and approaching zero, which agrees with almost all the peripheral carbons of the carbon skeleton of C48 being occupied; and the intensity of the characteristic peaks of the stretch vibration of C=O is increasing and approaching C5H9NO2.
In general, the IR spectra of 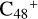 and C5H9NO2 are relatively different from the newly formed PAH-organic molecule clusters (from
and C5H9NO2 are relatively different from the newly formed PAH-organic molecule clusters (from 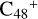 to
to 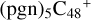 ). Some vibrational modes were preserved, while others are not, and some newly vibrational modes are formed. These spectra reveal the complexity of PAH-organic molecule cluster structure and the complexity of their infrared spectra, which also illustrates the important role of the complex organic molecules in the carbon skeleton and the connection type of clusters. Hence, the structure of clusters initially formed is diverse. Their role on the spectra profile contribution and the spectral features for tentative detections of PAH-organic molecule clusters in light of the graphene carbon cluster and organic molecule IR spectra in the ISM is warranted.
). Some vibrational modes were preserved, while others are not, and some newly vibrational modes are formed. These spectra reveal the complexity of PAH-organic molecule cluster structure and the complexity of their infrared spectra, which also illustrates the important role of the complex organic molecules in the carbon skeleton and the connection type of clusters. Hence, the structure of clusters initially formed is diverse. Their role on the spectra profile contribution and the spectral features for tentative detections of PAH-organic molecule clusters in light of the graphene carbon cluster and organic molecule IR spectra in the ISM is warranted.
 |
Fig. 8 Computed vibrational normal modes are represented: panel A is the IR spectrum of |
4 Astronomical implications
Prebiotic molecules are defined as molecules that are thought to be involved in the processes leading to the origin of life (Herbst & van Dishoeck 2009). Moreover, PAH molecules and their derivatives play an important role in the evolution network of prebiotic molecules in the ISM of of galaxies (Puget & Leger 1989; Allamandola 2011; Tielens 2013). The experiments in combination with the theoretical calculation results obtained in this work suggest that new large particles can be built up under the promotion of a strong irradiation field, yielding an interesting array of prebiotic molecules through aromatic-based compound (e.g., PAHs or graphenes) reactions with complex organic species (e.g., pyroglutamic acid, proline, and pyroglu-taminol) (Hu et al. 2021a). Organic molecules contain highly reactive reaction centers because of the functional groups, facilitating molecular adduct aggregation; more isomers are formed in the PAH-organic cluster cation systems. PAH-organic molecule cluster cations’ binding energy is relatively high, and even more large isomers (e.g., 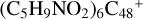 and (C5H9NO2)6DDC+) are therefore formed. The building block formation mechanisms provide a possible explanation for the formation of large complex prebiotic compounds in space (Tielens 2013).
and (C5H9NO2)6DDC+) are therefore formed. The building block formation mechanisms provide a possible explanation for the formation of large complex prebiotic compounds in space (Tielens 2013).
The relative activity of these species is very important in the ion-molecular reaction formation process and reaction evolution network, which affects the abundance ratio of formed species (Jäger et al. 2009; Montillaud et al. 2013; Zhen 2019; Yang et al. 2021). In the ISM, dehydrogenated PAHs or graphenes appear in the places where ultraviolet photons dominated them (Montillaud et al. 2013; Croiset et al. 2016). Organic molecules would have low abundances in the UV-rich environments (Sorrell 2001; Herbst & van Dishoeck 2009; Woods et al. 2012). Instead, the complex molecular inventory of hot cores and hot corinos is dominated by saturated molecules such as methanol, methyl formate (HCOOCH3), dimethyl ether (CH3OCH3), and propionitrile (CH3CH2CN), which can reach fractional abundances of 10−7, and also complex organic molecules would be expected in hot cores/hot corinos (Sorrell 2001; Herbst & van Dishoeck 2009; Woods et al. 2012). Although, no complex molecules have yet been detected in proto-planetary disks, either in the gas or in the ice (Herbst & van Dishoeck 2009). The molecule abundance spatial distributions of PAH species and the organic molecules do not overlap with their different distributions, which constraints the ISM’s cluster formation. Nevertheless, species formed by the reaction between PAH cations, graphene carbon cluster cations, and organic molecules may still be involved in the ISM (Herbst & van Dishoeck 2009).
We emphasize that the new clusters produced in this contribution range from 61 to 140 atoms or ~2–3 nm in size, which provides an approach for dust particle formation. Moreover, the building-block formation mechanisms (gas-phase condensation) provide a possible process for interpreting the formation of large prebiotic molecules in space (Allamandola 2011; Tielens 2013). Prebiotic compounds may be brought to the Earth by meteorites (e.g., Pizzarello & Huang 2005; Ehrenfreund & Charnley 2000). Carbonaceous meteorites contain numerous soluble organic compounds; many of these organic compounds contained familiar biochemical species (such as amino acids, fatty acids, purines, pyrimidines, and sugars; Cronin & Chang 1993; Botta & Bada 2002; Snyder 2006; Furukawa et al. 2019; Yang et al. 2021). All the complex species discussed above are produced through ion-molecules collision reactions. Thus, it seems reasonable to hypothesize that in the ISM, many of these compounds may first be incorporated as small dust or into small dust through gas-phase condensation by ion-molecules collision reactions. The small dust grows into large dust, then into or formed as comets and meteorites (Dunk et al. 2013; Delaunay et al. 2015; Zhen 2019; Gavilan et al. 2020).
5 Conclusions
In conclusion, theoretical calculations show that these newly formed graphene carbon cluster organic molecular cluster species can exist in a very stable way in the interstellar space simulated under laboratory conditions. The newly formed molecular clusters are a group of molecular clusters with a very complex molecular configuration, which suggests these clusters can play a catalytic role in the formation of prebiotic species in the ISM. The theoretical studies indicate that the interstellar-chemistry importance of ion-molecular reaction synthesis routes for complexity, and suggests that gas-phase interstellar matter can directly lead to large complex organic derivatives during bottom-up growth (in building block pathways). The production of such an interesting array of prebiotic molecules provides a deeper understanding of the evolutionary process of prebiotic molecules in space. Our results also reveal that free organic molecules can accumulate on small dust grains in the ISM (e.g., large PAHs or graphenes), which supports the suggestion that prebiotic molecules could be delivered to the early Earth by cometary dust, meteorites, or interplanetary dust particles.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC, Grant Nos. 41625013, 12073027, and 21827804), the Pre-research Project on Civil Aerospace Technologies (D020202) of the Chinese National Space Administration, and the Fundamental Research Funds for the Central Universities of China (WK3410000019). The theoretical calculations were performed at the Supercomputing Center of China’s University of Science and Technology.
References
- Allamandola, L.J., Tielens, A.G.G.M., & Barker, J.R. 1989, ApJS, 71, 733 [NASA ADS] [CrossRef] [Google Scholar]
- Allamandola, L.J., Joblin, C., & Tielens, A.G.G.M. 2011, EAS Pub. Ser., 46, 305 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Becke, A.D. 1992, J. Chem. Phys., 96, 2155 [NASA ADS] [CrossRef] [Google Scholar]
- Becker, L., Bada, J.L., Winans, R.E., & Bunch, T.E. 1994, Nature, 372, 507 [NASA ADS] [CrossRef] [Google Scholar]
- Berné, O., Joblin, C., Deville, Y., et al. 2007, A&A, 469, 575 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Berné, O., Montillaud, J., & Joblin, C. 2015, A&A, 577, A133 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Boersma, C., Bauschlicher, C.W. Jr., Ricca, A., et al. 2014, ApJS, 211, 8 [NASA ADS] [CrossRef] [Google Scholar]
- Botta, O., & Bada, J.L. 2002, Surv. Geophys. 23, 411 [NASA ADS] [CrossRef] [Google Scholar]
- Candian, A., Zhen, J., & Tielens, A.G.G.M. 2018, Phys. Today, 71, 38 [NASA ADS] [CrossRef] [Google Scholar]
- Castellanos, P., Candian, A., Zhen, J., et al. 2018, A&A, 616, A166 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cooper, G., Kimmich, N., Belisle, W., et al. 2001, Nature, 414, 879 [NASA ADS] [CrossRef] [Google Scholar]
- Croiset, B.A., Candian, A., Berné, O., & Tielens, A.G.G.M. 2016, A&A, 590, A26 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cronin, J.R., Chang, S. 1993, The Chemistry of Lifes Origin (The Netherlands: Springer) [Google Scholar]
- Delaunay, R., Gatchell, M., Rousseau, P., et al. 2015, J. Phys. Chem. Lett., 6, 1536 [CrossRef] [Google Scholar]
- Doucet, C., Habart, E., Pantin, E., et al. 2007, A&A, 470, 625 [CrossRef] [EDP Sciences] [Google Scholar]
- Dulieu, F., Nguyen, T., Congiu, E., et al. 2019, MNRAS, 484, L119 [NASA ADS] [CrossRef] [Google Scholar]
- Dunk, P.W., Adjizian, J.J., Kaiser, N.K., et al. 2013, Proc. Natl. Acad. Sci., 110, 18081 [NASA ADS] [CrossRef] [Google Scholar]
- Ehrenfreund, P., & Charnley, S.B. 2000, ARA&A, 38, 42 [Google Scholar]
- Engel, M.H., & Macko, S.A. 1997, Nature, 389, 265 [NASA ADS] [CrossRef] [Google Scholar]
- Favre, C., Fedele, D., Semenov, D., et al. 2018, ApJ, 862, L2 [NASA ADS] [CrossRef] [Google Scholar]
- Frisch, M.J., Trucks, G.W., Schlegel, H.B., et al. 2016, Gaussian 16 Revision e. 01 (USA: Gaussian Inc.) [Google Scholar]
- Furukawa, Y., Chikaraishi, Y., Ohkouchi, N., et al. 2019, Proc. Natl. Acad. Sci., 116, 24440 [NASA ADS] [CrossRef] [Google Scholar]
- Gavilan, L., Bejaoui, S., Haggmark, M., et al. 2020, ApJ, 889, 101 [NASA ADS] [CrossRef] [Google Scholar]
- Glavin, D.P., Alexander, C.M., Aponte, J.C., et al. 2018, in Primitive Meteorites and Asteroids, The Origin and Evolution of Organic Matter in Carbonaceous Chondrites and Links to their Parent Bodies, eds. N. Abreu (Amsterdam: Elsevier), 205 [Google Scholar]
- Grimme, S., Ehrlich, S., & Goerigk, L. 2011, J. Comput. Chem., 32, 1456 [CrossRef] [Google Scholar]
- Habart, E., Natta, A., Testi, L., & Carbillet, M. 2006, A&A, 449, 1067 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Herbst, E., & van Dishoeck, E.F. 2009, ARA&A, 47, 427 [NASA ADS] [CrossRef] [Google Scholar]
- Hollenbach, D.J., & Tielens, A.G.G.M. 1999, Rev. Mod. Phys., 71, 173 [NASA ADS] [CrossRef] [Google Scholar]
- Hu, X., Yang, Y., Chen, Y., Zhen, J., & Qin, L. 2021a, A&A, 656, A80 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Hu, X., Zhang, D., Yang, Y., et al. 2021b, ApJ, 918, 81 [NASA ADS] [CrossRef] [Google Scholar]
- Jäger, C., Huisken, F., Mutschke, H., Llamas Jansa, I., & Henning, T. 2009, ApJ, 696, 706 [CrossRef] [Google Scholar]
- Lee, C., Yang, W., & Parr, R.G. 1988, Phys. Rev. B, 37, 785 [Google Scholar]
- Lifshitz, C. 2000, IJMS, 200, 423 [NASA ADS] [Google Scholar]
- McGuire, B.A., Loomis, R.A., Burkhardt, A.M., et al. 2021, Science, 371, 1265 [NASA ADS] [CrossRef] [Google Scholar]
- Montillaud, J., Joblin, C., & Toublanc, D. 2013, A&A, 552, A15 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Pilleri, P., Joblin, C., Boulanger, F., et al. 2015, A&A, 577, A16 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Pizzarello, S., & Huang, Y. 2005, Geochim. Cosmochim. Acta, 69, 599 [CrossRef] [Google Scholar]
- Puget, J.L., & Leger, A. 1989, ARA&A, 27, 161 [NASA ADS] [CrossRef] [Google Scholar]
- Rapacioli, M., Joblin, C., & Boissel, P. 2005, A&A, 429, 193 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Rapacioli, M., Calvo, F., Joblin, C., et al. 2006, A&A, 460, 519 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Rhee, Y.M., Lee, T.J., Gudipati, M.S., Allamandola, L.J. & Head-Gordon, M. 2007, Proc. Natl. Acad. Sci., 104, 5274 [NASA ADS] [CrossRef] [Google Scholar]
- Sellgren, K. 1984, ApJ, 277, 623 [Google Scholar]
- Sephton, M.A., & Botta, O. 2008, Space Sci. Rev., 135, 25 [NASA ADS] [CrossRef] [Google Scholar]
- Snyder, L.E. 1997, Origins Life Evol. Biosph., 27, 115 [NASA ADS] [CrossRef] [Google Scholar]
- Snyder, L.E. 2006, Proc. Natl. Acad. Sci., 103, 12243 [NASA ADS] [CrossRef] [Google Scholar]
- Sorrell, W.H. 2001, ApJ, 555, L129 [NASA ADS] [CrossRef] [Google Scholar]
- Tielens, A.G.G.M. 2013, Rev. Mod. Phys., 85, 1021 [NASA ADS] [CrossRef] [Google Scholar]
- Van Diedenhoven, B., Peeters, E., Van Kerckhoven, C., et al. 2004, ApJ, 611, 928 [NASA ADS] [CrossRef] [Google Scholar]
- Woods, P.M., Kelly, G., Viti, S., et al. 2012, ApJ, 750, 19 [NASA ADS] [CrossRef] [Google Scholar]
- Woon, D.E. 2008, The astrochymist, http://www.astrochynrist.org [Google Scholar]
- Yang, Y., Zhang, C., Hu, et al. 2021, MNRAS, 508, 3009 [NASA ADS] [CrossRef] [Google Scholar]
- Zhang, W., Si, Y., Zhen, J., et al. 2019, ApJ, 872, 38 [NASA ADS] [CrossRef] [Google Scholar]
- Zhen, J. 2019, A&A, 623, A102 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Zhen, J., Castellanos, P., Paardekooper, D.M., Linnartz, H., & Tielens, A.G.G.M. 2014, ApJ, 797, L30 [CrossRef] [Google Scholar]
Databases, e.g., http://www.astrochymist.org, (Wood 2008); or the Cologne Database for Molecular Spectroscopy, https://cdms.astro.uni-koeln.de/classic/molecules
All Figures
 |
Fig. 1 Molecular geometry of graphene carbon cluster cations |
| In the text | |
 |
Fig. 2 Reaction pathways and the optimized structures of |
| In the text | |
 |
Fig. 3 Reaction pathways and the optimized structures of |
| In the text | |
 |
Fig. 4 Panel A: reaction pathways and optimized structures of |
| In the text | |
 |
Fig. 5 Panel A: reaction pathways and optimized structures of |
| In the text | |
 |
Fig. 6 Reaction pathways and the optimized structures of |
| In the text | |
 |
Fig. 7 Expanded tree (in building block pathways) shows the trunk and branches of these various formation pathways for these clusters, |
| In the text | |
 |
Fig. 8 Computed vibrational normal modes are represented: panel A is the IR spectrum of |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.