We have analyzed seven interplanetary dust particles (IDPs) using an infrared microscope with the aim of comparing the 3.4
A&A 433, 979-995 (2005)
DOI: 10.1051/0004-6361:20041605
G. Matrajt1 - G. M. Muñoz Caro1 - E. Dartois1 - L. d'Hendecourt1 - D. Deboffle1 - J. Borg1
Institut d'Astrophysique Spatiale, Bât. 121, Université Paris-Sud, 91405 Orsay Cedex, France
Received 7 July 2004 / Accepted 3 December 2004
Abstract
We have analyzed seven interplanetary dust particles (IDPs) using an
infrared microscope with the aim of comparing the 3.4 ![]() m feature to the
same feature observed in the diffuse interstellar medium (DISM) and of searching for
additional absorption features due to organic matter. We have developed
an in situ extraction method based on the etching of the silicate
phases with hydrofluoric acid, which allowed a better detection of the organic
fraction. Six out of the seven particles studied present a well resolved 3.4
m feature to the
same feature observed in the diffuse interstellar medium (DISM) and of searching for
additional absorption features due to organic matter. We have developed
an in situ extraction method based on the etching of the silicate
phases with hydrofluoric acid, which allowed a better detection of the organic
fraction. Six out of the seven particles studied present a well resolved 3.4 ![]() m
feature corresponding to aliphatic chains. Some of these particles also present a
carbonyl group around 1700 cm-1 due to ketone and, probably
also, carboxylic acid compounds.
Here we
report the first determination of the column densities of the
aliphatic and aromatic fractions in IDPs, as well as other functional
groups. With the asymmetric CH3 and CH2column densities we calculated the CH2/CH3 ratios of the IDPs. The average ratio
(CH2/CH
m
feature corresponding to aliphatic chains. Some of these particles also present a
carbonyl group around 1700 cm-1 due to ketone and, probably
also, carboxylic acid compounds.
Here we
report the first determination of the column densities of the
aliphatic and aromatic fractions in IDPs, as well as other functional
groups. With the asymmetric CH3 and CH2column densities we calculated the CH2/CH3 ratios of the IDPs. The average ratio
(CH2/CH
![]() )
is clearly larger than the one measured for the DISM (2.2),
indicating that the aliphatic chains in IDPs are longer (or less ramified) than in the DISM.
The 3.4
)
is clearly larger than the one measured for the DISM (2.2),
indicating that the aliphatic chains in IDPs are longer (or less ramified) than in the DISM.
The 3.4 ![]() m feature of IDPs is significantly narrower than in the DISM, which suggests that
the aliphatic fraction in IDPs is made of relatively simpler and
less ramified compounds. We conclude that the carbonaceous compounds found in these IDPs have
been formed or evolved in a different environment from the DISM.
m feature of IDPs is significantly narrower than in the DISM, which suggests that
the aliphatic fraction in IDPs is made of relatively simpler and
less ramified compounds. We conclude that the carbonaceous compounds found in these IDPs have
been formed or evolved in a different environment from the DISM.
Key words: interplanetary medium - ISM: molecules - astrochemistry - astrobiology - infrared: ISM
Interplanetary dust particles (IDPs) are extraterrestrial particles, also called cosmic dust, collected in the stratosphere by high-altitude aircrafts of the collection facility established by NASA. These particles are composed of different mineral phases, mainly silicates, and by a carbonaceous material which frequently forms the matrix in which the mineral grains are distributed (Bradley et al. 1984; Brownlee 1978).
Several infrared investigations of IDPs and the comparison of their spectra to infrared astrophysical data have been done in the past (Molster et al. 2003; Bradley et al. 1999; Sandford & Walker 1985; Keller et al. 2002; Molster et al. 2001; Keller & Flynn 2003). Infrared investigation of the carbonaceous matter in IDPs has also been performed (Flynn et al. 1998, 2002, 2003, 2004). In particular, Flynn et al. (1998,2002) showed the evidence for the first time for the presence of aliphatic hydrocarbons and of a ketone group, by detecting with an FTIR microscope the CH2, CH3 and C=O stretching functional groups in acid-etched IDPs. Also, Flynn et al. (2003) measured the CH2/CH3 ratio in several IDPs and compared it to the DISM.
In this work we study quantitatively the 3.4 ![]() m aliphatic feature
of IDPs, as well as other absorption features due to functional
groups in organics (in particular the carbonyl (C=O) and the OH stretching features),
by examining seven IDPs using an FTIR microscope. We also give a constraint on the
abundance of aromatic compounds
in IDPs. We first examine their
silicate component. Then we dissolve the silicates using hydrofluoric acid and
we re-examine the particle after the
acid-etching to study the organic residue.
The results are discussed on the basis of the
comparison of the organic feature at 3.4
m aliphatic feature
of IDPs, as well as other absorption features due to functional
groups in organics (in particular the carbonyl (C=O) and the OH stretching features),
by examining seven IDPs using an FTIR microscope. We also give a constraint on the
abundance of aromatic compounds
in IDPs. We first examine their
silicate component. Then we dissolve the silicates using hydrofluoric acid and
we re-examine the particle after the
acid-etching to study the organic residue.
The results are discussed on the basis of the
comparison of the organic feature at 3.4 ![]() m of IDPs with
the 3.4
m of IDPs with
the 3.4 ![]() m feature observed in the DISM.
m feature observed in the DISM.
We analyzed 7 IDPs. Four of them (L2021-K2; L2011-F2; L2021-K3; L2036-E19) were provided by the NASA-JSC curation facility. The first three originate from "clusters'', that formed each from a single fluffy particle that broke up into fragments upon impact on the collector surface, and are therefore known as cluster particles. L2011-F2 belonged to cluster 22, L2021-K2 belonged to cluster 4 and L2021-K3 belonged to cluster 5. L2036-E19 is not a cluster particle.
The other three IDPs (W7116B-U; W7116B-N and W7116B-Y) were extracted from one of the collectors, named W7116, carried by the NASA aircraft. The collector was examined under an optical microscope in a laminar-flow 10 000-class clean room. Black particles were removed using glass needles and paint brush hairs mounted in a micromanipulator. Because the collectors are coated with silicone oil to better decelerate the particles hitting the surface, the extracted particles are usually covered with silicone oil. Therefore, we rinsed them with hexane (purchased from Aldrich) several times in order to remove the excess of silicone oil. We then transferred them to a mount to be further characterized by Scanning Electron Microscopy (SEM). Energy-Dispersive X-ray (EDX) analyses were performed for each particle using a 20 kV accelerating voltage Jeol 35C microscope equipped with a Voyager 4 X-ray analysis system with ThermoNoran light element X-ray detector. The EDX data (not shown) were used to select chondritic particles, since it has been determined that "all stratospheric particles satisfying the chondritic composition criteria are extraterrestrial'' (Bradley et al. 1988; Sandford & Walker 1985).
IDPs are dominated by anhydrous and hydrated silicates, spinnels, carbonates, oxides and sulfides (Bradley et al. 1988; Fraundorf 1981) and a few percent of organic matter, such as PAHs (Clemett et al. 1993,1999) and aliphatic compounds (Flynn et al. 2003,2004). Some of the constituent minerals have strong absorption in the infrared region 650-4000 cm-1, which may interfere with the detection of the organic features. In some regions of the spectrum they even mask them. To remove most of this interference, we have developed a micrometer scale etching technique that removes in situ most of the silicate fraction, carbonates and sulfides, preserving the acid-insoluble organic matter. This technique, which is based on the hydrofluoric acid dissolution methodology used to prepare acid insoluble organic residues on meteorites (such as has been done in Orgueil or Murchison (Robert & Epstein 1982; Smith & Kaplan 1970; Gardinier et al. 2000)), is similar to the technique developed by Brownlee et al. (2000) and used on micro-scales by Flynn et al. (2002, 2004) for the analysis of organics in IDPs. However, the main difference with this methodology is that in our procedure we perform the etching in situ and over the entire particle, allowing therefore the analysis of the organics present throughout the whole volume of the particle.
The HF attack is performed as follows: after careful hexane cleaning of all the samples, the particles were crushed,
following the methodology described by Raynal et al. (2000),
on a
KRS-5 (Thallium Bromoiodide) window. This type of window is water-insoluble and
does not dissolve in HF (whereas the frequently used KBr does).
A first infrared analysis is performed on each sample to determine the kind of
silicates present in the particle and therefore its type.
Then, each sample is exposed for a few hours to a
hydrofluoric acid drop (48 wt![]() in water, 99.99%, purchased from Aldrich) in an Ar or N2 atmosphere to avoid oxidation.
This technique allows the removal of most (sometimes all) of
the silicates present in the particle very efficiently (Flynn et al. 2002; Brownlee et al. 2000) and
does not perturb or modify the organics that are acid-insoluble (Robert & Epstein 1982; Gardinier et al. 2000).
However, it is known that acid hydrolysis breaks peptidic bonds in proteins and
dissociates
certain organic molecules such as amines.
Therefore, we studied the possible effects of HF treatment on several organic
standards which were relevant for the identification of the features due to organics in IDPs, and
we found no significant changes in the band shapes and positions
(Sect. 3.3)
in water, 99.99%, purchased from Aldrich) in an Ar or N2 atmosphere to avoid oxidation.
This technique allows the removal of most (sometimes all) of
the silicates present in the particle very efficiently (Flynn et al. 2002; Brownlee et al. 2000) and
does not perturb or modify the organics that are acid-insoluble (Robert & Epstein 1982; Gardinier et al. 2000).
However, it is known that acid hydrolysis breaks peptidic bonds in proteins and
dissociates
certain organic molecules such as amines.
Therefore, we studied the possible effects of HF treatment on several organic
standards which were relevant for the identification of the features due to organics in IDPs, and
we found no significant changes in the band shapes and positions
(Sect. 3.3)
![\begin{figure}
\par\begin{tabular}{cc}
\par ORIGINAL & BASE LINE CORRECTED \\
&...
...width=7.9cm,height=5.7cm,clip]{1605fg1f.ps}\\
\par\end{tabular}\par\end{figure}](/articles/aa/full/2005/15/aa1605/Timg6.gif) |
Figure 1: Spectra of the W7116B particles studied before HF dissolution. The left column shows the original spectra with the corresponding baseline, the right column shows the same spectra after baseline subtraction. |
| Open with DEXTER | |
![\begin{figure}
\par\begin{tabular}{cc}
\par ORIGINAL & BASE LINE CORRECTED \\
&...
...idth=7.80cm,height=5.5cm,clip]{1605fg2h.ps}\\
\par\end{tabular}\par\end{figure}](/articles/aa/full/2005/15/aa1605/Timg7.gif) |
Figure 2: Spectra of the NASA particles studied before HF dissolution. The left column shows the original spectra with the corresponding baseline, the right column shows the same spectra after baseline subtraction. |
| Open with DEXTER | |
We used Fourier Transform InfraRed spectroscopy, employing
a Nicolet Magna-IR 560 ESP spectrometer attached to
a Nicolet Nicplan infrared microscope, in line with a Synchrotron
Radiation Source located on line SU5 at the Laboratoire pour l'Utilisation
du Rayonnement Electromagnétique (LURE) at the University of Paris-Sud, Orsay, France.
The spectrometer is equipped with a KBr beamsplitter and a nitrogen-cooled mercury-cadmium-tellure
(MCT) detector working in the 4000 to 650 cm-1 (2.5 to 15 ![]() m) spectral range.
The microscope uses a Schwarzchild-Cassegrain objective (32
m) spectral range.
The microscope uses a Schwarzchild-Cassegrain objective (32![]() )
and a condenser (10
)
and a condenser (10![]() )
in the transmission mode. Besides the Synchrotron light source, the
standard light source of the instrument, a conventional black body-like source
(Globar) is available.
Further details about this instrument and the advantages of
both light sources can be found in Raynal et al. (2000).
For apertures above 6
)
in the transmission mode. Besides the Synchrotron light source, the
standard light source of the instrument, a conventional black body-like source
(Globar) is available.
Further details about this instrument and the advantages of
both light sources can be found in Raynal et al. (2000).
For apertures above 6 ![]() m2, no significant differences were observed between the spectra obtained with the internal Globar source and the Synchrotron source. Moreover, the Globar was more stable than the Synchrotron source. Therefore, most of
the analyses described here were performed using the internal Globar source, more frequently available.
m2, no significant differences were observed between the spectra obtained with the internal Globar source and the Synchrotron source. Moreover, the Globar was more stable than the Synchrotron source. Therefore, most of
the analyses described here were performed using the internal Globar source, more frequently available.
Standards of organic compounds were used to better identify the carrier of the peak around 1700 cm-1. They were all purchased from Aldrich. We used a ketone [R-C(=O)-R'] in solid state at room temperature, 2-hexadodecanone (C16H32O), and three carboxylic acids [R-COOH]: stearic acid (C18H36O2), adipic acid (C6H10O4) and succinic acid (C4H6O4).
In order to enhance the signal-to-noise ratio and minimize background fluctuations due to purge variations, 25 acquisitions of 256 scans each at the resolution of 2 cm-1 were recorded for each sample and then averaged. However, the baseline in each spectrum is not always easy to establish because it varies according to the thickness of the sample. For example, when the sample is too thick, the IR radiation suffers diffraction, producing a steep decrease in the baseline around the 1000 cm-1 region (see for an example the spectrum of particle W7116B-Y in Fig. 1). The baseline also varies according to the type of material the IR beam passes through, and for silicates the baseline is hardly horizontal (see particle L2021-K3 in Fig. 2 for a representative example). We have corrected our spectra by subtracting a spline baseline. The baseline is the result of a spline fit, generated by an in-house IDL program, to a number of points given manually. Our code also provides the positions and areas of the spectral features. The original spectra of the IDPs are presented in Figs. 1, 2, 4 and 5: the panels in the left column show the original spectra with the corresponding baseline and the panels in the right column are the spectra after baseline subtraction.
Figures 1 and 2 show the spectra of all the particles analyzed during this study before the HF etching. The spectra of the same particles after HF treatment are shown in Figs. 4 and 5.
Many particles (W7116B-U, W7116B-Y, L2011-F2, L2021-K3, L2021-K2 and L2036-E19) present remaining silicone oil, with
characteristic peaks at 806-800, 1095-1080, 1260, 2963 cm-1 (see the
silicone oil spectrum in Fig. 8).
The particle W7116B-Y has also peaks at 1457-1450 cm-1, corresponding to carbonates.
There are also some particles (W7116B-N, L2011-F2 and L2021-K3) with peaks near ![]() 1400 cm-1 whose interpretation is
at present unknown.
In Table 1 all the peaks observed before the HF dissolution are listed, together
with their ascribed vibration mode and assignment.
1400 cm-1 whose interpretation is
at present unknown.
In Table 1 all the peaks observed before the HF dissolution are listed, together
with their ascribed vibration mode and assignment.
Table 1: Peaks present in the spectra before the HF treatment.
The analyses of the particles before HF dissolution make it possible to see the silicate signature of each IDP. Sandford & Walker (1985) described FTIR analyses of several IDPs and found that IDPs can be grouped in three spectral classes referred to as olivines (Oli), layer-lattice silicates (LLS) and pyroxenes (Px) after the minerals that provide the best match to the dust spectra. Following this description, we have compared each one of the spectra obtained during our work with the representative spectra of each of the three classes established by Sandford & Walker (1985), and classified all the particles studied (Table 2). From this classification we found that four out of seven particles (W7116B-Y; W7116B-N; L2021-K3 and L2021-K2) seem to have a mixture of anhydrous and hydrated layer-lattice silicates, being therefore hydrated IDPs. The remaining particles are composed of anhydrous minerals, either only pyroxene (L2036-E19) or a mixture of olivine and pyroxene (W7116B-U and L2011-F2), and thus are anhydrous IDPs.
Table 2: IDPs classification.
![\begin{figure}
\par\resizebox{8.8cm}{!}{\includegraphics[clip]{1605fig3.eps}}\par
\par\end{figure}](/articles/aa/full/2005/15/aa1605/Timg11.gif) |
Figure 3: The aliphatic peaks of the region 3000-2800 cm-1, for the seven IDPs before (dashed line) and after (solid line) HF treatment. Some spectra have been multiplicated by a factor (indicated in the right side) for intercomparison. |
| Open with DEXTER | |
Besides the peaks related to silicates, most of the particles also display peaks related to organic compounds that are frequently found in two main regions: 3000-2800 cm-1 (aliphatic C-H stretching modes) and 1700-1300 cm-1(the assignment of these is difficult due to the presence of water in phyllosilicates (OH-bending mode) and carbonate features in the same region). In the region 3000-2800 cm-1 four main peaks are visible which correspond to the CH2 and CH3 symmetric and asymmetric stretching peaks at about 2850, 2865, 2920 and 2958 cm-1 (Fig. 3). Most of the particles show only three prominents peaks, since the CH3symmetric vibration, which is the weakest band, is blended with the CH2 band at 2850 cm-1. The particle W7116B-U
Table 3: Band strengths of molecules used for integration.
does not present distinguishable peaks but rather a broad band. The particle L2011-F2 does not present any of these four peaks; instead it displays a peak at 2960 cm-1, which is not organic but a remnant of the silicone oil. Moreover, the L2021-K2 particle presents peaks at 1375 and 1583 cm-1 which can be related to the COO- stretch of a carboxylic acid salt (see Sect. 3.4).
Table 4: Aliphatic column density (noted N[3.4], in the region 3000-2800 cm-1) and silicate column density (N[10], around 1000 cm-1) of the IDPs before the HF treatment, calculated after baseline correction. See text for error estimates.
In Table 4 are shown the areas of
the features corresponding to the Si-O
stretch of silicates (between 1100-700 cm-1, "10 ![]() m band'') and the C-H stretch of
aliphatics (between 3000-2800 cm-1, "3.4
m band'') and the C-H stretch of
aliphatics (between 3000-2800 cm-1, "3.4 ![]() m band'') that were
calculated after a baseline correction of each spectrum.
It should be noted that the exact values of the areas
calculated are baseline dependent, but are evaluated in the
same way for each IDP, allowing intercomparison.
The estimated error of the area is calculated by comparing the result
obtained with a spline baseline (shown in Fig. 1) with the result using
a linear baseline for the local continuum. The error so obtained is
less than 30% of the calculated area for the broad OH stretch and no more than 15% for the other bands.
The column densities for aliphatics (N[3.4], in aliphatic C atoms/cm2)
and silicates (N[10], in Si atoms/cm2) and the ratio N[3.4]/N[10], in
aliphatic C-atoms/Si-atoms are also given. These column densities were determined using the
band strengths for different molecules (see Table 3) and
the formula:
m band'') that were
calculated after a baseline correction of each spectrum.
It should be noted that the exact values of the areas
calculated are baseline dependent, but are evaluated in the
same way for each IDP, allowing intercomparison.
The estimated error of the area is calculated by comparing the result
obtained with a spline baseline (shown in Fig. 1) with the result using
a linear baseline for the local continuum. The error so obtained is
less than 30% of the calculated area for the broad OH stretch and no more than 15% for the other bands.
The column densities for aliphatics (N[3.4], in aliphatic C atoms/cm2)
and silicates (N[10], in Si atoms/cm2) and the ratio N[3.4]/N[10], in
aliphatic C-atoms/Si-atoms are also given. These column densities were determined using the
band strengths for different molecules (see Table 3) and
the formula:
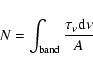 |
(1) |
![\begin{figure}
\par\begin{tabular}{cc}
\par ORIGINAL & BASE LINE CORRECTED \\
&...
...8.5cm,,height=5.8cm,clip]{1605fg4f.ps}\\
\par\end{tabular}\par
\par\end{figure}](/articles/aa/full/2005/15/aa1605/Timg38.gif) |
Figure 4: Spectra of the W7116B particles studied after HF dissolution. The left column shows the original spectra with the corresponding baseline, the right column shows the same spectra after baseline subtraction. Please note the difference in scale with respect to the spectra obtained before HF (Fig. 1). |
| Open with DEXTER | |
![\begin{figure}
\par\begin{tabular}{cc}
\par ORIGINAL & BASE LINE CORRECTED \\
\...
...width=7.8cm,height=5.5cm]{1605fg5h.ps}\\
\par\end{tabular}\par
\par\end{figure}](/articles/aa/full/2005/15/aa1605/Timg39.gif) |
Figure 5: Spectra of the NASA particles studied after HF dissolution. The left column shows the original spectra with the corresponding baseline, the right column shows the same spectra after baseline subtraction Please note the difference in scale with respect to the spectra obtained before HF (Fig. 2). |
| Open with DEXTER | |
The band strength of the SiO bond (A[SiO]) has been determined as follows:
The measurements of the column densities of the bands due to the CH (N[3.4]) and SiO (N[10]) stretchings, and their comparison (N[3.4]/N[10], Table 4) shows that the number of Si atoms is larger than the number of C atoms in these IDPs, with one exception: W7116B-Y. The proportions, however, are not similar, showing that some particles contain more organics than others.
In Table 5 the main peaks observed after the HF dissolution are shown, together with the vibration mode they may represent and their potential identifications. Figures 4 and 5 show the spectra of the particles after the HF treatment.
Table 5: Peaks present in the spectra after the HF treatment.
Many of the peaks that were present before the HF
treatment have been reduced (or disappeared) after the acid dissolution
(790-820; 870-905; 1010-1020; 1050-1100; 1400-1450; 2963 cm-1).
Moreover, some peaks
that were not clearly distinguished before the HF treatment can now be found
(1700-1714; 1450-1500; ![]() 1600; 3250 cm-1).
There are also some peaks that were already present before the HF treatment
which are still observed
after the acid dissolution
(800-806; 1080-1095; 1260; 1375; 1400-1460; 1583; 2800-3000; 3100-3600 cm-1).
In a general manner, the HF treatment has dissolved most of the silicate
fraction off the particles, as can be seen in Table 6. The
column densities for both silicates and aliphatics
are also shown in Table 6. Table 8 reports the areas and column
densities
of the bands at 3600-2400, 1700, and 1585 cm-1 attributed to the OH, C=O and COO-functional groups. The areas and column densities of the band at 1600 cm-1, assigned to
aromatic compounds, are also indicated. These values should be taken only as indicative, as they may present large uncertainties that are caused by the uncertainties in the values of the band strengths, the decomposition of the bands needed for integration, and possible effects due to the HF treatment required for its detection. In the regions where two bands were blended we used a Gaussian decomposition for the integration of the areas.
1600; 3250 cm-1).
There are also some peaks that were already present before the HF treatment
which are still observed
after the acid dissolution
(800-806; 1080-1095; 1260; 1375; 1400-1460; 1583; 2800-3000; 3100-3600 cm-1).
In a general manner, the HF treatment has dissolved most of the silicate
fraction off the particles, as can be seen in Table 6. The
column densities for both silicates and aliphatics
are also shown in Table 6. Table 8 reports the areas and column
densities
of the bands at 3600-2400, 1700, and 1585 cm-1 attributed to the OH, C=O and COO-functional groups. The areas and column densities of the band at 1600 cm-1, assigned to
aromatic compounds, are also indicated. These values should be taken only as indicative, as they may present large uncertainties that are caused by the uncertainties in the values of the band strengths, the decomposition of the bands needed for integration, and possible effects due to the HF treatment required for its detection. In the regions where two bands were blended we used a Gaussian decomposition for the integration of the areas.
Table 6:
Aliphatic column density (
![]() ], see
Table 7) in the region
2800-3000 cm-1 and silicate column density (N[10]) around 1000 cm-1)
of the IDPs after the HF treatment, calculated after a baseline correction. See text for error estimates.
], see
Table 7) in the region
2800-3000 cm-1 and silicate column density (N[10]) around 1000 cm-1)
of the IDPs after the HF treatment, calculated after a baseline correction. See text for error estimates.
After the HF treatment all particles (except L2036-E19) have three peaks corresponding to the
CH2 symmetric vibration (at ![]() 2850 cm-1), the CH2 asymmetric vibration
(at
2850 cm-1), the CH2 asymmetric vibration
(at ![]() 2922 cm-1) and the CH3 asymmetric vibration (at
2922 cm-1) and the CH3 asymmetric vibration (at ![]() 2958 cm-1).
The weak CH3 symmetric vibration
(at
2958 cm-1).
The weak CH3 symmetric vibration
(at ![]() 2865 cm-1), is generally blended with the
2865 cm-1), is generally blended with the ![]() 2850 cm-1 band.
2850 cm-1 band.
The particle L2036-E19 has lost the aliphatic peaks
that were present before the HF treatment.
The particle W7116B-Y lost part of
its aliphatic component, as shown by the decrease of the area of the 3.4 ![]() m feature
([3.4] area, Table 6).
This decrease in the aliphatic fraction can be explained by
the removal of an acid-soluble organic compound whose origin is at present unknown.
Because this compound washes out easily with HF, it could also be
attributed to an organic contaminant that was sticking on the surface of the particle.
Therefore, the excess of organic in these two particles
may be considered to be either indigenous or a surface contamination.
Particle L2011-F2 presented a prominent peak at 2963 cm-1 due to the remaining silicone
oil, which was removed by the HF treatment.
m feature
([3.4] area, Table 6).
This decrease in the aliphatic fraction can be explained by
the removal of an acid-soluble organic compound whose origin is at present unknown.
Because this compound washes out easily with HF, it could also be
attributed to an organic contaminant that was sticking on the surface of the particle.
Therefore, the excess of organic in these two particles
may be considered to be either indigenous or a surface contamination.
Particle L2011-F2 presented a prominent peak at 2963 cm-1 due to the remaining silicone
oil, which was removed by the HF treatment.
The column densities of the CH3 and CH2 groups, N[CH3] and N[CH2], were calculated by fitting the 2958 and 2922 cm-1 bands with two Gaussians, using the integrated band strengths given in Sect. 3.1.2. The fitting procedure was tested with the spectrum measured toward GC IRS7, a probe of the diffuse interstellar medium, obtaining N[CH2]/N[CH3]=2.2, which is in agreement with the literature value of 2.0-2.5 (Pendleton et al. 1994; Sandford et al. 1991). The values of N[CH3], N[CH2], and N[CH2]/N[CH3], are given in Table 7.
Table 7:
Column densities of the asymmetric
aliphatic peaks in the region
2800-3000 cm-1 present in the IDPs spectra after the HF treatment, and
of the IRS 7 source of the Galactic Center. The estimated error of these values, due
to spectral noise and the quality of the Gaussian fit, is
![]() 10%.
10%.
The average ratio CH2/CH3 calculated for the anhydrous
particles is 3.0 and the one calculated for the hydrated particles
is 4.1.
The difference between these two ratios is probably not significant as
the number of IDPs studied remains limited. The shape and bandwidth of the
3.4 ![]() m feature is quite similar in both types of IDPs.
Moreover, the values of N[CH2] and N[CH3] do not differ
significantly, indicating that anhydrous and hydrated IDPs contain roughly similar
concentrations of aliphatic hydrocarbons.
Both observations suggest that the aliphatic C-H bonding in the two
types of particles is similar.
m feature is quite similar in both types of IDPs.
Moreover, the values of N[CH2] and N[CH3] do not differ
significantly, indicating that anhydrous and hydrated IDPs contain roughly similar
concentrations of aliphatic hydrocarbons.
Both observations suggest that the aliphatic C-H bonding in the two
types of particles is similar.
Some IDPs (see Table 8) show a peak around 1700 cm-1, that is attributed to
the carbonyl stretch. This peak is not clearly present in the
spectra taken before the HF treatment. However, after the acid etching
the peak becomes clear and sharp in most of the particles, suggesting that
this peak was partially masked by the H-O-H bending feature at 1660 cm-1,
characteristic of the structural water. The comparison of N[3.4]
(Table 6) to N[1700] (Table 8) shows that
the number of C=O bonds is for some particles only slightly lower than the number of C atoms
in aliphatic chains.
Based on its position (![]() 1712 cm-1), the carbonyl group can be attributed to
either a ketone or a carboxylic acid. In order to find out whether this
infrared absorption is produced by a ketone or by a carboxylic acid, we have
compared our spectra with the spectra of a representative compound of each of these
two groups: for ketones we used 2-hexadodecanone; for carboxylic acids
we used stearic, succinic, and adipic acids (Fig. 6).
We exposed the standards to the same HF treatment used for the
other samples, in order to test if the features at 1700 cm-1 and
between 3000-2800 cm-1 change in profile, position or height after the acid treatment.
It appeared that the hydrofluoric acid did not affect significantly any of the compounds used as
standards, because both
the peaks at 1700 cm-1 and around 3000 cm-1 have similar band shapes and positions after
the HF treatment. For adipic and succinic acid there is a decrease in the absorbance, probably due
to wash-out. These observations support the idea that the feature at 1700 cm-1observed in some IDPs belongs to a carbonyl group and is not the result
of a shift of a pre-existing peak after the HF treatment.
1712 cm-1), the carbonyl group can be attributed to
either a ketone or a carboxylic acid. In order to find out whether this
infrared absorption is produced by a ketone or by a carboxylic acid, we have
compared our spectra with the spectra of a representative compound of each of these
two groups: for ketones we used 2-hexadodecanone; for carboxylic acids
we used stearic, succinic, and adipic acids (Fig. 6).
We exposed the standards to the same HF treatment used for the
other samples, in order to test if the features at 1700 cm-1 and
between 3000-2800 cm-1 change in profile, position or height after the acid treatment.
It appeared that the hydrofluoric acid did not affect significantly any of the compounds used as
standards, because both
the peaks at 1700 cm-1 and around 3000 cm-1 have similar band shapes and positions after
the HF treatment. For adipic and succinic acid there is a decrease in the absorbance, probably due
to wash-out. These observations support the idea that the feature at 1700 cm-1observed in some IDPs belongs to a carbonyl group and is not the result
of a shift of a pre-existing peak after the HF treatment.
 |
Figure 6: Spectra of the standards 2-hexadodecanone, stearic, succinic and adipic acids before (solid line) and after (dashed line) HF treatment. |
| Open with DEXTER | |
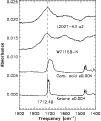 |
Figure 7: Spectral region near 1700 cm-1 for the standards 2-hexadodecanone and stearic acid and their comparison with W7116B-N and L2021-K3 post HF particles. |
| Open with DEXTER | |
We compared some spectra of IDPs (W7116B-N; L2021-K3) after the acid etching to the two standards of ketone and carboxylic acid (Fig. 7). The precise position of the carbonyl group in the IDPs lies between 1706 and 1712 cm-1, which best matches the position of the carbonyl in a ketone. However, these IDPs also show a very broad band between 3500-2300 cm-1, which is characteristic of the OH stretch in carboxylic acids. Therefore, in this peak there is probably also some contribution from carboxylic acids. These features are found in both anhydrous and hydrated IDPs, suggesting that the presence of carboxylic acids and ketones is not silicate dependent.
The spectrum of particle L2021-K2 before hydrolysis (Fig. 2) is particularly interesting. It displays two bands at 1583 and 1376 cm-1, which are compatible in shape and position with the COO- anti-symmetric and symmetric stretchings of ammonium salts of carboxylic acids. The spectrum after hydrolysis (Fig. 5) displays the same peaks, although their absorbance has decreased and they are blended with other peaks. A new peak appears at 1691 cm-1, which is probably due to carboxylic acids. This supports the presence of carboxylic acid salts, since the hydrolysis of these compounds would lead to the formation of carboxylic acids by the substitution of the cation in the salt (NH4+ for ammonium salts) by a H. The comparison of N[3.4] (Table 6) to N[1585] (Table 8) shows that for particle L2021-K2 the number of COO- groups is double the number of aliphatic C atoms. Two other particles, L2011-F2 and W7116B-Y, might also contain carboxylic acid salts (see Table 8).
The relatively weak CH stretch band of aromatic hydrocarbons at
![]() 3030 cm-1 is not observed in the samples studied.
A better tracer of aromatics is the band at 1600 cm-1 (ring stretch), as this band has
a much larger intrinsic absorption than the 3030 cm-1 band and does not depend on the degree
of hydrogenation of the aromatics. Three IDPs (L2021-K3, W7116B-U and W7116B-N) present a feature centered
at 1600 cm-1, which could be due to an aromatic component.
3030 cm-1 is not observed in the samples studied.
A better tracer of aromatics is the band at 1600 cm-1 (ring stretch), as this band has
a much larger intrinsic absorption than the 3030 cm-1 band and does not depend on the degree
of hydrogenation of the aromatics. Three IDPs (L2021-K3, W7116B-U and W7116B-N) present a feature centered
at 1600 cm-1, which could be due to an aromatic component.
The aromatic peaks observed in these IDPs could be attributed to PAHs,
since PAHs have already been detected in some IDPs using other analytical techniques (Clemett et al. 1993).
Assuming a band strength of
![]() cm C atom-1 (for average neutral PAHs,
Schutte et al. 1998) these IDPs would have between
cm C atom-1 (for average neutral PAHs,
Schutte et al. 1998) these IDPs would have between ![]() 78 and 114 times more carbon in
aromatic than in aliphatic form. The other IDPs, however, do not show clear peaks around
1600 cm-1, indicating that the amount of aromatic components in our IDPs is very
variable.
78 and 114 times more carbon in
aromatic than in aliphatic form. The other IDPs, however, do not show clear peaks around
1600 cm-1, indicating that the amount of aromatic components in our IDPs is very
variable.
Table 8: Integration of other organic peaks present in the spectra after the HF treatment.
It could be argued that some of the organic peaks observed during these analyses are indeed contamination that could have come from several sources: silicone oil, hexane used to clean it, the KRS-5 window, the terrestrial atmosphere. In order to investigate the possibility of contamination, we conducted analyses in several materials that we have used as blanks. In Fig. 8 we show the spectra of all the blanks used during this work.
![\begin{figure}
\par\resizebox{8.5cm}{!}{\includegraphics[clip]{1605fig8.ps}}
\par\end{figure}](/articles/aa/full/2005/15/aa1605/Timg91.gif) |
Figure 8: Spectra of the materials used as blanks during this work: hexane; KRS-5; olivine; silicone oil; W7116-200; W7116B-K. For the latter two only the spectra after HF dissolution are shown. |
| Open with DEXTER | |
HF was used previously to dissolve the silicates in order to investigate the carbonaceous component of IDPs with FTIR (Flynn et al. 2002,2004). In these previous works, transmission electron microscope (TEM) sections of IDPs were hydrolyzed instead of the entire particle. Thus, if the organics are heterogeneously distributed throughout the particle, there is no way to obtain the right section for the analysis. This is the reason why in this work we have performed an in situ acid dissolution of the entire crushed particle. This presents the advantage that the silicates composing the particle are almost entirely dissolved, and thus that all the acid-insoluble carbonaceous matter of the particle is concentrated, improving the detection and resolution of the aliphatic bands. The HF treatment will be further discussed in Sect. 4.1.
Some IDP spectra present peaks at 1260, 1100 and 803 cm-1 which are consistent with the main peaks of silicone oil (see Fig. 8), indicating that the hexane in some cases does not entirely remove the silicone oil from the particle. In most cases, the remaining silicone oil is reduced or totally washed out with the HF (see Tables 1 and 5). However, in a few cases a tiny peak of silicone oil at 1260 cm-1 is still present. Flynn et al. (2002, 2004) also found some unremoved silicone oil in their samples, which suggests that this problem is not intrinsic to our method of etching but rather to the difficulty of completely removing the silicone oil by dissolution with organic solvents (hexane) or acids (HF).
To estimate the contribution of the silicone oil peaks to the areas
of the integrated bands is not
straightforward, as often the silicone oil peaks overlap
with both the silicate and the organic features we are interested in.
However, from integration of the 1260 cm-1 peak due to silicone
oil, and using the relative area values of the silicone oil peaks as
deduced from the standard spectrum of silicone oil shown in
Fig. 8, we can give a rough estimate of the contribution of
silicone oil to the observed areas of the silicate and aliphatic
CH-stretching bands, respectively in the 10 and 3.4 ![]() m regions.
In particular, it was found that the area of the 2963 cm-1 band
is 3 times smaller than the area of the 1260 cm-1 band, which made it possible
to estimate roughly the contribution of silicone oil to the 3.4
m regions.
In particular, it was found that the area of the 2963 cm-1 band
is 3 times smaller than the area of the 1260 cm-1 band, which made it possible
to estimate roughly the contribution of silicone oil to the 3.4 ![]() m
band.
For both bands, this contribution is of
the order of 10% or lower before hydrolisis, as for IDP W7116B-N, except
for particles W7116B-Y and L2011-F2 which contain more silicone oil. After
hydrolysis, little silicone oil remains in the spectra, except for
particles W7116B-Y and W7116B-U (as observed by comparison of Figs. 1 and 2
with Figs. 4 and 5).
The presence of silicone oil is only
problematic for the determination of the CH2/CH3 ratio, as the
position of the 2963 cm-1 silicone oil peak is expected to overlap
with the 2958 cm-1 band (CH3 asymmetric vibration). However,
except for IDPs W7116B-Y and W7116B-U, the CH2/CH3 values after
hydrolysis should not be affected, as little silicone oil remains.
m
band.
For both bands, this contribution is of
the order of 10% or lower before hydrolisis, as for IDP W7116B-N, except
for particles W7116B-Y and L2011-F2 which contain more silicone oil. After
hydrolysis, little silicone oil remains in the spectra, except for
particles W7116B-Y and W7116B-U (as observed by comparison of Figs. 1 and 2
with Figs. 4 and 5).
The presence of silicone oil is only
problematic for the determination of the CH2/CH3 ratio, as the
position of the 2963 cm-1 silicone oil peak is expected to overlap
with the 2958 cm-1 band (CH3 asymmetric vibration). However,
except for IDPs W7116B-Y and W7116B-U, the CH2/CH3 values after
hydrolysis should not be affected, as little silicone oil remains.
Some of the blanks (silicone oil, hexane and
terrestrial particles) used during this work to control
our results for contamination were also employed by
Flynn et al. (2004). In this previous work, the analyses
of blanks did not show
any aliphatic signature for evaporated hexane or silicone oil. However, the terrestrial particles
showed a weak C-H absorption feature at ![]() 2960 cm-1 and positions near
but not identical to those seen in their IDPs. This suggests that terrestrial
contamination does not affect very much
the FTIR analyses since the terrestrial C-H signatures have positions and strengths different
from IDPs.
2960 cm-1 and positions near
but not identical to those seen in their IDPs. This suggests that terrestrial
contamination does not affect very much
the FTIR analyses since the terrestrial C-H signatures have positions and strengths different
from IDPs.
The identification of organics in most of the particles
we analyzed showed that both anhydrous and hydrated IDPs contain organics.
This was also observed by Flynn et al. (2003,2004) and Raynal et al. (2001).
It was also observed that
the positions of the aliphatic peaks
around the 3.4 ![]() m region vary slightly from one particle to another.
However, these variations are still in the ranges of the known CH2 and CH3 peak positions.
m region vary slightly from one particle to another.
However, these variations are still in the ranges of the known CH2 and CH3 peak positions.
The column densities of the aliphatic peaks calculated after the HF treatment (Table 7) show that the aliphatic CH2 and CH3 groups are present in different quantities in each IDP, regardless of kind (anhydrous or hydrated). This observation was also made by Flynn et al. (2003) and suggests that some IDPs are richer in aliphatics than others. This also suggests that the aliphatic abundance is not dependent on the kind of silicate that constitutes the IDP particle.
Besides aliphatic chains, some IDPs also have a carbonyl group (C=O). This functional group was identified in IDPs during previous studies using FTIR (Flynn et al. 2002,2003,2004) and using carbon and oxygen XANES (Keller et al. 2002; Flynn et al. 2003; Reffner et al. 1994). These previous results corroborate our finding of a C=O functional group in most of the IDPs studied.
The use of HF during this work made it possible to improve the recognition of several organic features. Indeed, diffusion is enhanced by the different diffraction indexes coming from both the silicates and the carbonaceous material present in the particle. Removal of the silicates reduces this effect.
The values of the silicate column densities (N[10], see Tables 4 and 6) show that for all IDPs, except W7116B-Y, N[10] clearly diminishes after the HF treatment (from about 2.5 to 50 times), showing that the in situ HF treatment is very efficient in dissolving the silicates. Furthermore, for most IDPs the bands in the 3000 cm-1 region are much better resolved, which allowed the calculation of the column densities corresponding to the CH2 and CH3 groups. Moreover, after the HF etching the bands in the 1000-1700 cm-1 region, such as the carbonyl peak and the C=C bonds, are now visible, as there is little overlap with the bands due to silicates and other minerals. This has allowed the detection of a ketone and/or a carboxylic acid compound in most of the particles, and also of the presence of the 1600 cm-1 band ascribed to aromatics. Thus, the dissolution method with HF developed in this work has been very useful for improving the detection of several organic features in these particles. However, it is necessary to test the hydrolysis protocol with molecular standards, as there might be organic components that could be affected by the hydrolysis.
Several investigations of the
acid extract of the carbonaceous chondrites Murchison and Orgueil
have been performed to date(Ehrenfreund et al. 1991; de Vries et al. 1993; Gardinier et al. 2000).
All of them showed that both meteorites present four main peaks
at 2960, 2925, 2875 and 2855 cm-1, with the latter two
bands blended into one single band around 2865 cm-1.
In addition, the aliphatic fraction of the carbonaceous chondrite
Tagish Lake was also investigated (Matrajt et al. 2004) and it was found
that the meteorite is dominated by long aliphatic chains, with a
CH2/CH3 ratio of ![]() 7.3.
We have compared the spectra of our IDPs with the ones
available from Murchison and Orgueil.
This comparison shows
that the band widths of the 3.4
7.3.
We have compared the spectra of our IDPs with the ones
available from Murchison and Orgueil.
This comparison shows
that the band widths of the 3.4 ![]() m feature
in IDPs are narrower than in the meteorites (by about 30-40 cm-1),
suggesting that the aliphatic chains in IDPs are made of simpler compounds.
Moreover, the CH2/CH
3 = 3.7 ratio of the seven IDPs studied is larger than the value
of
m feature
in IDPs are narrower than in the meteorites (by about 30-40 cm-1),
suggesting that the aliphatic chains in IDPs are made of simpler compounds.
Moreover, the CH2/CH
3 = 3.7 ratio of the seven IDPs studied is larger than the value
of ![]() 2 corresponding to Murchison and Orgueil, but shorter than the value of
2 corresponding to Murchison and Orgueil, but shorter than the value of ![]() 7
corresponding to Tagish Lake. It can thus be concluded that
the aliphatic chains in IDPs are longer (or less
ramified) than in Murchison and Orgueil but shorter
(or more ramified) than in Tagish Lake, showing that
the aliphatic material in IDPs is
different from that found in
carbonaceous chondrites. This suggests that
the carbonaceous material found in both
kinds of objects was formed (or was processed) in different environments
and/or under different conditions, and therefore has a different origin.
7
corresponding to Tagish Lake. It can thus be concluded that
the aliphatic chains in IDPs are longer (or less
ramified) than in Murchison and Orgueil but shorter
(or more ramified) than in Tagish Lake, showing that
the aliphatic material in IDPs is
different from that found in
carbonaceous chondrites. This suggests that
the carbonaceous material found in both
kinds of objects was formed (or was processed) in different environments
and/or under different conditions, and therefore has a different origin.
Flynn et al. (2004) and Keller et al. (2004) compared the carbonyl group present in Murchison to the one observed in IDPs and found that it has a similar position in both objects. The position of the carbonyl found in our IDPs is also similar to that of the carbonyl in Murchison, suggesting that both carbonaceous chondrites and IDPs have a fraction of oxygen-rich organics harboring a carbonyl functional group.
To investigate the origin (molecular cloud material, diffuse interstellar material, ...)
of the organic phases observed in these IDPs,
we compared the acid extract of three of the IDPs, the
W7116B-U, W7116B-Y and L2021-K2 particles, to the spectrum of the GC IRS 7 source,
a probe of the diffuse interstellar medium (Fig. 9).
This comparison clearly shows that the IDP spectra
and the DISM spectrum are different in several significant ways.
First, the IDP spectra are dominated by the CH2 peaks (at ![]() 2923
and 2855 cm-1) whereas the DISM spectrum has CH2 (at
2923
and 2855 cm-1) whereas the DISM spectrum has CH2 (at ![]() 2923 cm-1)
and CH3 (at
2923 cm-1)
and CH3 (at ![]() 2958 cm-1) peaks
of similar strength, and, unlike for IDPs, the CH3 peak at
2958 cm-1) peaks
of similar strength, and, unlike for IDPs, the CH3 peak at ![]() 2865 cm-1 is clearly
present. Indeed the computed average CH2/CH3 ratio for IDPs is 3.7
(see Table 7 and Sect. 3.2.2), suggesting that in the DISM the aliphatics
are either more ramified or shorter than in the IDPs.
Flynn et al. (2003) also found that the average ratio in their IDPs,
ranging from
2865 cm-1 is clearly
present. Indeed the computed average CH2/CH3 ratio for IDPs is 3.7
(see Table 7 and Sect. 3.2.2), suggesting that in the DISM the aliphatics
are either more ramified or shorter than in the IDPs.
Flynn et al. (2003) also found that the average ratio in their IDPs,
ranging from
![]() in the hydrated IDPs to
in the hydrated IDPs to
![]() in the anhydrous IDPs, was
higher than in the DISM.
Second, the 3.4
in the anhydrous IDPs, was
higher than in the DISM.
Second, the 3.4 ![]() m band in the IDP
spectra is narrower than the band corresponding to dust absorption
in the DISM, suggesting that the molecules in the IDPs are simpler. All together, the aliphatic
component in the DISM is more complex and ramified than in IDPs, suggesting
that the two carbonaceous compounds were formed in different
environments or under different conditions and at different times in the Universe.
m band in the IDP
spectra is narrower than the band corresponding to dust absorption
in the DISM, suggesting that the molecules in the IDPs are simpler. All together, the aliphatic
component in the DISM is more complex and ramified than in IDPs, suggesting
that the two carbonaceous compounds were formed in different
environments or under different conditions and at different times in the Universe.
![\begin{figure}
\par\resizebox{8.8cm}{!}{\includegraphics[clip]{1605fig9.ps}}
\par\end{figure}](/articles/aa/full/2005/15/aa1605/Timg94.gif) |
Figure 9: Comparison of the spectrum of the particles W7116B-Y (hydrated) before HF, L2021-K2 (cluster, hydrated) after HF and W7116B-U (anhydrous) after HF to the IRS 7 source (Galactic Center). Only the 2800-3100 cm-1 region is shown. The Galactic Center source spectrum has been obtained from the Infrared Space Observatory (ISO) Data Center. |
| Open with DEXTER | |
It is worth noting that the L2021-K2 particle is a cluster particle
(Table 2). Messenger (2000) found significant excesses of
D, 15N and 16O in cluster particles and deduced that these
particles contained "preserved interstellar material'' and therefore
were the most primitive class of Solar System materials.
However, the 3.4 ![]() m feature in cluster IDPs is similar to that of the
other IDPs studied, and it is
different from the 3.4
m feature in cluster IDPs is similar to that of the
other IDPs studied, and it is
different from the 3.4 ![]() m feature of the diffuse interstellar medium.
Therefore, the aliphatic hydrocarbons
in cluster IDPs are not preserved
diffuse interstellar materials but rather
materials that formed in a different environment from the diffuse interstellar medium, such as
dense
clouds, or materials that were re-processed in the Solar System.
m feature of the diffuse interstellar medium.
Therefore, the aliphatic hydrocarbons
in cluster IDPs are not preserved
diffuse interstellar materials but rather
materials that formed in a different environment from the diffuse interstellar medium, such as
dense
clouds, or materials that were re-processed in the Solar System.
It could be argued that the ratios measured during this study in the aliphatics of IDPs have been altered during the experimental process of etching with HF. We have shown that, after etching, several organic peaks that were masked by the silicate peaks are now visible or better resolved, showing that the etching is helping to detect more organic material.
In Fig. 3 are shown the CH2 and CH3 peaks
(before and after the HF treatment)
corresponding to the region 3000-2800 cm-1.
The peaks in the spectra before the HF etching (dashed line)
are not well resolved in most of the particles. However, particle W7116B-Y
shows an intense and well-resolved 3.4 ![]() m feature previous to hydrolysis. Comparison
of this band before and after HF treatment shows that the shape, band positions and CH2/CH3ratio are not modified by the hydrolysis.
Furthermore, in Fig. 6 are shown the spectra of four organic standards
before and after having been treated with HF. In this figure the
peaks in the region 3000-2800 cm-1 are well resolved
and it is clear that they have not changed (neither in position, nor
in relative height) after the HF treatment.
We can conclude that the HF etching is not altering
the CH2/CH3 ratios measured, suggesting
that these ratios in IDPs are originally different
from that observed in the DISM.
m feature previous to hydrolysis. Comparison
of this band before and after HF treatment shows that the shape, band positions and CH2/CH3ratio are not modified by the hydrolysis.
Furthermore, in Fig. 6 are shown the spectra of four organic standards
before and after having been treated with HF. In this figure the
peaks in the region 3000-2800 cm-1 are well resolved
and it is clear that they have not changed (neither in position, nor
in relative height) after the HF treatment.
We can conclude that the HF etching is not altering
the CH2/CH3 ratios measured, suggesting
that these ratios in IDPs are originally different
from that observed in the DISM.
It could also be argued that the CH2/CH3 ratios measured in these IDPs are different from the diffuse ISM because of the heating during the atmospheric entry of these particles. The high temperatures to which these particles are exposed during their entry would have altered or evaporated some molecules, changing the overall CH2/CH3 ratio of the remaining organic material. Models of atmospheric entry heating of IDPs suggest that IDPs are heated for a few seconds to 600-800 K during their atmospheric entry (Flynn 1989). But there must be a gradient in the temperature to which the particle is really exposed, allowing the survival of volatile compounds, since ketones and carboxylic acids are found in IDP matrices, as we have demonstrated in the present work and as has been demonstrated during other studies (Flynn et al. 2003,2004). We have also demonstrated the presence of indigenous aliphatics in the matrices of these IDPs, and aliphatics do not survive at temperatures above 800-1000 K (Dartois, unpublished results). Wdowiak et al. (1988) performed heating experiments at several temperatures up to 800 K of the acid insoluble residue of the Orgueil carbonaceous chondrite. These experiments demonstrated that the evaporating temperature of the compounds forming the bands in the 3000 cm-1 region lies in the range 700-800 K. In these experiments, it was clearly shown that the peaks in this region do not change shape or relative height (see Fig. 1 of Wdowiak et al. 1988), they only decrease in size with temperature because of evaporation, suggesting that the ratio CH2/CH3 is not affected by temperature. Therefore, it is unlikely that the ratio CH2/CH3 measured in the IDPs was altered by atmospheric entry heating. However, more pulse-heating experiments are needed to completely rule out this possibility.
PAHs (Polycyclic Aromatic Hydrocarbons) are present in IDPs (Clemett et al. 1993; Allamandola et al. 1987).
Reactions transforming these PAHs could lead to the formation
of CH2 side groups. For example, the UV photolysis of PAHs
produces partially hydrogenated aromatic
hydrocarbons (Bernstein et al. 2002,1999) and alkylated PAHs (Mahajan et al. 2003).
However, a quantification of the PAH abundances in IDPs does not exist
to date. We found that three out of the seven IDPs showed a band at ![]() 1600 characteristic of
aromatics, which indicates that they contain at most
1600 characteristic of
aromatics, which indicates that they contain at most ![]() 78-114 times more carbon in aromatic than in aliphatic
form (see Sect. 3.5). However, the other IDPs did not present
a band at this position. Moreover, unlike aliphatic compounds, aromatic hydrocarbons
are not an ubiquitous component of the dust in the general DISM (Chiar et al. 2000).
Therefore, the mechanism described above
would take place only rarely and thus it is unlikely that
the 3.4
78-114 times more carbon in aromatic than in aliphatic
form (see Sect. 3.5). However, the other IDPs did not present
a band at this position. Moreover, unlike aliphatic compounds, aromatic hydrocarbons
are not an ubiquitous component of the dust in the general DISM (Chiar et al. 2000).
Therefore, the mechanism described above
would take place only rarely and thus it is unlikely that
the 3.4 ![]() m feature measured in these IDPs would originate from aromatic precursors.
m feature measured in these IDPs would originate from aromatic precursors.
Alternatively, the presence of Hn-PAHs (PAHs that have a few additional hydrogen atoms attached, Bernstein et al. 1996; Schutte et al. 1993) in IDPs, would lead to an increase on strength of the features characteristic of the symmetric and asymmetric C-H stretching vibrations of the CH2 groups (Bernstein et al. 1996). However, these features in Hn-PAHs have their positions slightly shifted from the normal position in aliphatics, and usually fall in the vicinity of 2940 and 2850 cm-1 (Bernstein et al. 1996). In the IDPs we have studied during this work the symmetric and asymmetric positions of the CH2 groups are well defined and constant: 2850 and 2922 cm-1, respectively. Therefore, it seems unlikely that the CH2 features observed in IDPs contain a significant contribution from the CH2 features typical of Hn-PAHs because the band near 2940 cm-1 is not observed (see Fig. 3). The absence of such a band also suggests that if Hn-PAHs are present in these IDPs, they are not linked to the aliphatic molecules. Therefore, it is unlikely that the ratio CH2/CH3 measured in the IDPs is different from the one observed in the DISM by the presence of PAHs. The observed difference must be attributed to the fact that the carbonaceous material found in these IDPs was formed in an environment different from the diffuse interstellar medium.
In this work we have analyzed seven interplanetary dust particles (IDPs) using an
infrared microscope. We have developed
an in situ extraction method based on the dissolution of the silicate
phases with hydrofluoric acid, which allowed the organic phases to be better
detected. We have found that six of the seven particles studied present a well resolved 3.4 ![]() m
feature which corresponds to aliphatic chains. Most of the particles analyzed
(L2021-K3, L2011-F2, W7116B-N, W7116B-Y and W7116B-U)
also presented a carbonyl group around 1700 cm-1, which appears to be due
to ketones with some contribution of carboxylic acids. Some particles (L2021-K3, W7116B-U and W7116B-N) show a band
around 1600 cm-1, which can be attributed to aromatics. We calculated the column densities
of functional groups and aromatics in the IDPs studied.
We determined the column densities
of the asymmetric CH3 and CH2 peaks in our IDPs and we calculated their CH2/CH3 ratios using
a Gaussian fit, obtaining an average of 3.7.
We did not observe a significant difference between the hydrated and the anhydrous
IDPs. Using the same fit we also re-determined the CH2/CH3 ratio
in the GC IRS 7 interstellar object, and found a value of 2.2. The comparison of this
value to the one obtained for the IDPs indicated that the aliphatic chains
in the IDPs are longer (or less ramified) than in the diffuse ISM.
Given that the 3.4
m
feature which corresponds to aliphatic chains. Most of the particles analyzed
(L2021-K3, L2011-F2, W7116B-N, W7116B-Y and W7116B-U)
also presented a carbonyl group around 1700 cm-1, which appears to be due
to ketones with some contribution of carboxylic acids. Some particles (L2021-K3, W7116B-U and W7116B-N) show a band
around 1600 cm-1, which can be attributed to aromatics. We calculated the column densities
of functional groups and aromatics in the IDPs studied.
We determined the column densities
of the asymmetric CH3 and CH2 peaks in our IDPs and we calculated their CH2/CH3 ratios using
a Gaussian fit, obtaining an average of 3.7.
We did not observe a significant difference between the hydrated and the anhydrous
IDPs. Using the same fit we also re-determined the CH2/CH3 ratio
in the GC IRS 7 interstellar object, and found a value of 2.2. The comparison of this
value to the one obtained for the IDPs indicated that the aliphatic chains
in the IDPs are longer (or less ramified) than in the diffuse ISM.
Given that the 3.4 ![]() m band of organics in the diffuse ISM is significantly broader than the same band in IDPs,
we conclude that the organics in IDPs are simpler than the organics present in
the diffuse ISM, suggesting
that the carbonaceous
compound found in these IDPs has been formed in a different environment from the diffuse ISM.
m band of organics in the diffuse ISM is significantly broader than the same band in IDPs,
we conclude that the organics in IDPs are simpler than the organics present in
the diffuse ISM, suggesting
that the carbonaceous
compound found in these IDPs has been formed in a different environment from the diffuse ISM.
Studies concerning the origin and evolution of the organic fraction in IDPs are currently in process.
Acknowledgements
We acknowledge the CNES-INSU who provided post-doctoral support for G.M., and the Marie Curie fellowship program who provided post-doctoral support for G.M.M.C. We thank P. Dumas for his assistance during the FTIR analyses. We acknowledge the NASA curation facility (in particular M. Zolensky) who provided the samples used during this study.