Table 1:
Chemical surface reactions r assumed to form the solid materials s. The efficiency of the reaction is limited by the collision rate of the key species, which has the lowest abundance among the reactants. The notation
 in the rhs column means that only every second collision (and sticking) event initiates one reaction (see
in the rhs column means that only every second collision (and sticking) event initiates one reaction (see
 in Eqs. (4) and (10)). Data sources for the supersaturation ratios (and saturation vapour pressures): (1) Helling & Woitke (2006); (2) Nuth & Ferguson (2006); (3) Sharp & Huebner (1990).
in Eqs. (4) and (10)). Data sources for the supersaturation ratios (and saturation vapour pressures): (1) Helling & Woitke (2006); (2) Nuth & Ferguson (2006); (3) Sharp & Huebner (1990).
| Index r |
Solid s |
Surface reaction |
Key species |
| 1 |
TiO2[s] |
TiO2
 TiO2[s]
TiO2[s] |
TiO2 |
| 2 |
rutile |
Ti + 2 H2O
 TiO2[s] + 2 H2
TiO2[s] + 2 H2 |
Ti |
| 3 |
(1) |
TiO + H2O
 TiO2[s] + H2
TiO2[s] + H2 |
TiO |
| 4 |
|
TiS + 2 H2O
 TiO2[s] + H2S + H2
TiO2[s] + H2S + H2 |
TiS |
| 5 |
SiO2[s] |
SiO2
 SiO2[s]
SiO2[s] |
SiO2 |
| 6 |
silica |
SiO + H2O
 SiO2[s] + H2
SiO2[s] + H2 |
SiO |
| 7 |
(3) |
SiS + 2 H2O
 SiO2[s] + H2S + H2
SiO2[s] + H2S + H2 |
SiS |
| 8 |
SiO[s] |
SiO
 SiO[s]
SiO[s] |
SiO |
| 9 |
silicon mono-oxide |
SiO2 + H2
 SiO[s] + H2O
SiO[s] + H2O |
SiO2 |
| 10 |
(2) |
SiS + H2O
 SiO[s] + H2S
SiO[s] + H2S |
SiS |
| 11 |
Fe[s] |
Fe
 Fe[s]
Fe[s] |
Fe |
| 12 |
solid iron |
FeO + H2
 Fe[s] + H2O
Fe[s] + H2O |
FeO |
| 13 |
(1) |
FeS + H2
 Fe[s] + H2S
Fe[s] + H2S |
FeS |
| 14 |
|
Fe(OH)2 + H2
 Fe[s] + 2 H2O
Fe[s] + 2 H2O |
Fe(OH)2 |
| 15 |
FeO[s] |
FeO
 FeO[s]
FeO[s] |
FeO |
| 16 |
iron (II) oxide |
Fe + H2O
 FeO[s] + H2
FeO[s] + H2 |
Fe |
| 17 |
(3) |
FeS + H2O
 FeO[s] + H2S
FeO[s] + H2S |
FeS |
| 18 |
|
Fe(OH)2
 FeO[s] + H2
FeO[s] + H2 |
Fe(OH)2 |
| 19 |
FeS[s] |
FeS
 FeS[s]
FeS[s] |
FeS |
| 20 |
iron sulphide |
Fe + H2S
 FeS[s] + H2
FeS[s] + H2 |
Fe |
| 21 |
(3) |
FeO + H2S
 FeS[s] + H2O
FeS[s] + H2O |
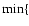 FeO, H2S FeO, H2S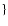 |
| 22 |
|
Fe(OH)2 + H2S
 FeS[s] + 2 H2O
FeS[s] + 2 H2O |
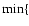 Fe(OH)2, H2S Fe(OH)2, H2S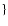 |
| 23 |
Fe2O3[s] |
2 Fe + 3 H2O
 Fe2O3[s] + 3 H2
Fe2O3[s] + 3 H2 |
 Fe Fe |
| 24 |
iron (III) oxide |
2 FeO + H2O
 Fe2O3[s] + H2
Fe2O3[s] + H2 |
 FeO FeO |
| 25 |
(3) |
2 FeS + 3 H2O
 Fe2O3[s] + 2 H2S + H2
Fe2O3[s] + 2 H2S + H2 |
 FeS FeS |
| 26 |
|
2 Fe(OH)2
 Fe2O3[s] + H2O + H2
Fe2O3[s] + H2O + H2 |
 Fe(OH)2 Fe(OH)2 |
| 27 |
MgO[s] |
MgO
 MgO[s]
MgO[s] |
MgO |
| 28 |
periclase |
Mg + H2O
 MgO[s] + H2
MgO[s] + H2 |
Mg |
| 29 |
(3) |
2 MgOH
 2 MgO[s] + H2
2 MgO[s] + H2 |
 MgOH MgOH |
| 30 |
|
Mg(OH)2
 MgO[s] + H2O
MgO[s] + H2O |
Mg(OH)2 |
| 31 |
MgSiO3[s] |
Mg + SiO + 2 H2O
 MgSiO3[s] + H2
MgSiO3[s] + H2 |
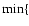 Mg, SiO Mg, SiO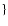 |
| 32 |
enstatite |
Mg + SiS + 3 H2O
 MgSiO3[s] + H2S + 2 H2
MgSiO3[s] + H2S + 2 H2 |
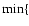 Mg, SiS Mg, SiS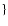 |
| 33 |
(3) |
2 MgOH + 2 SiO + 2 H2O
 2 MgSiO3[s] + 3 H2
2 MgSiO3[s] + 3 H2 |
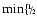 MgOH, MgOH,
 SiO SiO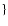 |
| 34 |
|
2 MgOH + 2 SiS + 2 H2O
 2 MgSiO3[s] + 2 H2S + 2 H2
2 MgSiO3[s] + 2 H2S + 2 H2 |
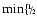 MgOH, MgOH,
 SiS SiS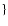 |
| 35 |
|
Mg(OH)2 + SiO
 2 MgSiO3[s] + H2
2 MgSiO3[s] + H2 |
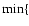 Mg(OH)2, SiO Mg(OH)2, SiO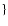 |
| 36 |
|
Mg(OH)2 + SiS + H2O
 MgSiO3[s] + H2S+ H2
MgSiO3[s] + H2S+ H2 |
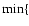 Mg(OH)2, SiS Mg(OH)2, SiS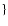 |
| 37 |
Mg2SiO4[s] |
2 Mg + SiO + 3 H2O
 Mg2SiO4[s] + 3 H2
Mg2SiO4[s] + 3 H2 |
 Mg, SiO Mg, SiO |
| 38 |
forsterite |
2 MgOH + SiO + H2O
 Mg2SiO4[s] + 2 H2
Mg2SiO4[s] + 2 H2 |
 MgOH, SiO MgOH, SiO |
| 39 |
(3) |
2 Mg(OH)2 + SiO
 Mg2SiO4[s] + H2O + H2
Mg2SiO4[s] + H2O + H2 |
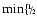 Mg(OH)2, SiO Mg(OH)2, SiO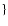 |
| 40 |
|
2 Mg + SiS + 4 H2O
 Mg2SiO4[s] + H2S + 3 H2
Mg2SiO4[s] + H2S + 3 H2 |
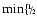 Mg, SiS} Mg, SiS} |
| 41 |
|
2 MgOH + SiS + 2 H2O
 Mg2SiO4[s] + H2S + 2 H2
Mg2SiO4[s] + H2S + 2 H2 |
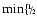 MgOH, SiS} MgOH, SiS} |
| 42 |
|
2 Mg(OH)2 + SiS
 Mg2SiO4[s] + H2 + H2S
Mg2SiO4[s] + H2 + H2S |
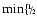 Mg(OH)2, SiS} Mg(OH)2, SiS} |
| 43 |
Al2O3[s] |
2 Al + 3 H2O
 Al2O3[s] + 3 H2
Al2O3[s] + 3 H2 |
 Al Al |
| 44 |
aluminia |
2 AlOH + H2O
 Al2O3[s] + 2 H2
Al2O3[s] + 2 H2 |
 AlOH AlOH |
| 45 |
(3) |
2 AlH + 3 H2O
 Al2O3[s] + 4 H2
Al2O3[s] + 4 H2 |
 AlH AlH |
| 46 |
|
Al2O + 2 H2O
 Al2O3[s] + 2 H2
Al2O3[s] + 2 H2 |
Al2O |
| 47 |
|
2 AlS + 3 H2O
 Al2O3[s] + 2 H2S + H2
Al2O3[s] + 2 H2S + H2 |
 AlS AlS |
| 48 |
|
2 AlO2H
 Al2O3[s] + H2O
Al2O3[s] + H2O |
 AlO2H AlO2H |
| 49 |
CaTiO3[s] |
Ca + TiO + 2 H2O
 CaTiO3[s] + 2 H2
CaTiO3[s] + 2 H2 |
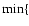 Ca, TiO Ca, TiO |
| 50 |
perovskite |
Ca + TiO2 + H2O
 CaTiO3[s] + H2
CaTiO3[s] + H2 |
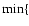 Ca, TiO Ca, TiO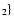 |
| 51 |
(3) |
Ca + Ti + 3 H2O
 CaTiO3[s] + 3 H2
CaTiO3[s] + 3 H2 |
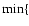 Ca, Ti Ca, Ti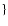 |
| 52 |
|
CaO + Ti + 2 H2O
 CaTiO3[s] + 2 H2
CaTiO3[s] + 2 H2 |
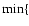 CaO, Ti CaO, Ti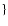 |
| 53 |
|
CaO + TiO + H2O
 CaTiO3[s] + H2
CaTiO3[s] + H2 |
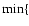 CaO, TiO CaO, TiO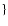 |
| 54 |
|
CaO + TiO2
 CaTiO3[s]
CaTiO3[s] |
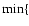 CaO, TiO CaO, TiO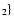 |
| 55 |
|
CaS + Ti + 3 H2O
 CaTiO3[s] + H2S + H2
CaTiO3[s] + H2S + H2 |
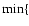 CaS, Ti CaS, Ti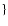 |
| 56 |
|
CaS + TiO + 2 H2O
 CaTiO3[s] + H2S + 2 H2
CaTiO3[s] + H2S + 2 H2 |
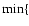 CaS, TiO CaS, TiO |
| 57 |
|
CaS + TiO2 + H2O
 CaTiO3[s] + H2S
CaTiO3[s] + H2S |
 CaS, TiO CaS, TiO |
| 58 |
|
Ca(OH)2 + Ti + H2O
 CaTiO3[s] + 2 H2
CaTiO3[s] + 2 H2 |
 Ca(OH)2, Ti Ca(OH)2, Ti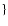 |
| 59 |
|
Ca(OH)2 + TiO
 CaTiO3[s] + H2
CaTiO3[s] + H2 |
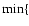 Ca(OH)2, TiO Ca(OH)2, TiO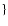 |
| 60 |
|
Ca(OH)2 + TiO2
 CaTiO3[s] + H2O
CaTiO3[s] + H2O |
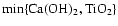 |