- ... grains
![[*]](/icons/foot_motif.gif)
- Appendices are only available in electronic form at http://www.edpsciences.org
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ... molecules
![[*]](/icons/foot_motif.gif)
- Hopping requires
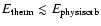 ,
where
,
where
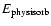 is the
binding energy of an alien ad-molecule on a solid surface. The
efficiency of the hopping process depends hence on the
temperature. If
is the
binding energy of an alien ad-molecule on a solid surface. The
efficiency of the hopping process depends hence on the
temperature. If
 is too small, the arriving molecules
can not move at the grains surface. If
is too small, the arriving molecules
can not move at the grains surface. If
 is too large,
the just arrived molecule recoils from the surface (Gail &
Sedlmayr 1984).
is too large,
the just arrived molecule recoils from the surface (Gail &
Sedlmayr 1984).
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ...1988)
![[*]](/icons/foot_motif.gif)
- Venables (1993) review such rate equation models
discussing several experimental studies to show how these concepts can be applied.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ... reactions
![[*]](/icons/foot_motif.gif)
- For molecules M and
grains S we define:
| type I reaction: |
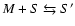 |
| type II reaction: |
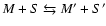 |
| type III reaction: |
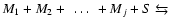 |
(
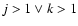 ) ) |
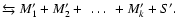 |
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ... equilibrium
![[*]](/icons/foot_motif.gif)
- Due to the high
densities in substellar atmospheres, deviations from thermal and
chemical equilibrium are unlikely to occur, unless the atmosphere is
illuminated from the outside. A fast collisional energy exchange
between dust and gas is assured and hence
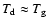 (see Fig. 4 in Paper II).
(see Fig. 4 in Paper II).
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ...2003)
![[*]](/icons/foot_motif.gif)
-
Note that e.g. Al2O3[s] can only be formed in the
presence of a pre-existing surface, but is not considered as nucleation
species due to the lack of a stable monomer Al2O3 in the gas phase
(see Patzer 2004).
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ... abundances
![[*]](/icons/foot_motif.gif)
- Figure 5 has been
calculated with the full chemical equilibrium code (14 elements and
155 molecules) which enables us to cross-check the reduced version of
the chemical equilibrium code used during the hydrodynamic
simulations.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ...)
![[*]](/icons/foot_motif.gif)
- Note the error in the
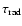 estimation in Eq. (25) in Paper I.
estimation in Eq. (25) in Paper I.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ...
![[*]](/icons/foot_motif.gif)
![[*]](/icons/foot_motif.gif)
- The exact value
depends on the definition of the cooling time-scale considered. If
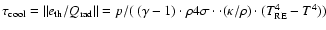 ,
it follows
,
it follows
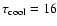 s and 0.53 s without and with dust,
respectively.
s and 0.53 s without and with dust,
respectively.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ...
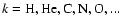 )
)![[*]](/icons/foot_motif.gif)
- This is different from Dominik et al.
(1993), who assumed that the element composition of the
gas resembles that of the solid, which we think is incorrect.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.