A&A 428, 117-120 (2004)
DOI: 10.1051/0004-6361:20041649
H. Liszt1 - R. Lucas2 - J. H. Black3
1 - National Radio Astronomy Observatory,
520 Edgemont Road,
Charlottesville, VA
22903-2475, USA
2 - Institut de Radioastronomie Millimétrique,
300 rue de la Piscine,
38406 Saint-Martin-d'Hères,
France
3 - Chalmers Centre for Astrophysics and Space Science,
Onsala Space Observatory,
Chalmers University of Technology,
43992 Onsala, Sweden
Received 12 July 2004 / Accepted 23 August 2004
Abstract
We used the Plateau de Bure Interferometer to search for ![]() 3mm
absorption lines of HOC+ from local diffuse and translucent clouds
occulting compact extragalactic mm-wave continuum sources. We detected
HOC+ in three directions with column densities only 70-120 times below
that of the HCO+ isomer, a factor 5-50 higher than typically
found in dense dark gas but comparable to recent observations of
dense photon-dominated regions. The observed amounts of HOC+
N(HOC+)/N(H2)
3mm
absorption lines of HOC+ from local diffuse and translucent clouds
occulting compact extragalactic mm-wave continuum sources. We detected
HOC+ in three directions with column densities only 70-120 times below
that of the HCO+ isomer, a factor 5-50 higher than typically
found in dense dark gas but comparable to recent observations of
dense photon-dominated regions. The observed amounts of HOC+
N(HOC+)/N(H2)
![]() can be made in quiescent
diffuse gas at thermal gas-kinetic rates if the H2O/OH ratio
is of order unity, in mild violation of extant observational limits.
can be made in quiescent
diffuse gas at thermal gas-kinetic rates if the H2O/OH ratio
is of order unity, in mild violation of extant observational limits.
Key words: ISM: molecules - astrochemistry
The roster of polyatomic molecules known to exist in diffuse
interstellar gas has been steadily enlarged over the last
fifteen years and now numbers nearly one dozen. Two of
these, C3 (Maier et al. 2001; Oka et al. 2003; Roueff et al. 2002) and H3+ (McCall et al. 2003) were recently discovered in
optical and near-infrared absorption spectra. The others
have accumulated gradually over the last twenty or so years
in cm-wave and mm-wave radiofrequency absorption data and
now include H2CO (Marscher et al. 1993; Nash 1990; Federman & Willson 1982; Moore & Marscher 1995; Liszt & Lucas 1995),
HCO+ (Lucas & Liszt 1996),
N3 (Nash 1990), C2H (Lucas & Liszt 2000), 3h2
(Cox et al. 1988; Lucas & Liszt 2000), HCN and HNC (Liszt & Lucas 2001) and
![]() S and HCS+ (Lucas & Liszt 2002).
S and HCS+ (Lucas & Liszt 2002).
Observationally, the polyatomics observed with radio techniques are often seen to be strongly and directly related to simpler species observed at optical wavelengths, i.e. HCO+ to OH (Liszt & Lucas 1996; Lucas & Liszt 1996) HCN and HNC to CN (Liszt & Lucas 2001) etc. These connections point to fundamental lacunae in our understanding of the chemistry of even the simplest species in diffuse clouds: simple models which reproduce the abundances of diatomics, however easily, invariably fall orders of magnitude short of explaining the related polyatomics. Conversely, the high abundances of some polyatomics transform other mysteries; the poorly-understood abundances of CO and CS in diffuse clouds are readily understandable given the recombination of the observed columns of HCO+ (Liszt & Lucas 2000) and HCS+ (Lucas & Liszt 2002) respectively.
The work described here rounds out to an even dozen the number of polyatomics known to exist in diffuse and translucent clouds. HOC+, the energetically-disfavored HCO+ isomer whose 89.4874 GHz J=1-0 transition we sought, was indeed detected along several of the sightlines studied in the other molecular species mentioned in the preceding paragraphs. The HCO+-HOC+ comparison is especially interesting now because both species have recently been detected over a wide range of physical conditions in dense gas: our new spectra reinforce an emerging view which relates molecular abundances in dense and diffuse PDR. Section 2 gives details of the observations, which are discussed in Sect. 3 and summarized in Sect. 4.
The 89.4874 GHz J=1-0 transition of HOC+ was observed at the Plateau de Bure at various times during the period 2000-2001 toward the five sources listed in Table 1. Profiles were taken with 39.1 kHz (0.13 kms-1) channel separation and 70 kHz resolution and subsequently once hanning smoothed, yielding spectra with the line/continuum rms values as listed in Table 1.
The column density in the lowest (J=0) level of a simple linear
molecule is related to the integrated optical depth of the J=1-0transition as
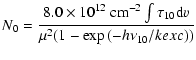 |
(1) |
With the exception of CO (Liszt & Lucas 1998), the molecules observed
at mm-wavelengths
in diffuse gas are well-described by assuming that the rotational
ladder is thermalized at the temperature of the cosmic microwave
background, in which case the total column density
can be calculated straightforwardly from observations of one
transition. We take the dipole moments of HOC+ and HCO+ as
2.8D and 3.93D, respectively, a slight departure from prior work
in which we used 4.07D for HCO+. Given these assumptions, one
has N(X)/
![]() for X = HOC+,
for X = HOC+,
![]() for X = HCO+ and
for X = HCO+ and
![]() for X = H13CO+.
for X = H13CO+.
Table 1: Background sources observed.
Table 2: Column densities.
The mm-wave absorption data are summarized in Tables 1-3 and
line profiles toward two sources are shown in Figs. 1 and 2. The
HCO+ and OH column densities are taken from our prior published
(Liszt & Lucas 1996; Lucas & Liszt 1996) and unpublished work; for B0415+379
(see Fig. 1) we assert N(HCO+) = ![]() N(H13CO+)
(Lucas & Liszt 1998) because of the high optical optical depth
in the main isotope. HOC+ is found to have relative abundances
HCO+:HOC+ = 70-120, HOC+:OH
N(H13CO+)
(Lucas & Liszt 1998) because of the high optical optical depth
in the main isotope. HOC+ is found to have relative abundances
HCO+:HOC+ = 70-120, HOC+:OH
![]() toward
three sources (see Table 3). The results obtained toward the
other two, B0528 and B1730, are not of great significance.
The relative abundance of OH, for which it is found that
X(OH) = n(OH)/n(H2)
toward
three sources (see Table 3). The results obtained toward the
other two, B0528 and B1730, are not of great significance.
The relative abundance of OH, for which it is found that
X(OH) = n(OH)/n(H2)
![]() along the four sightlines where both N(H2) and N(OH) have
been measured (Liszt & Lucas 2002), sets the relative abundance
scale for HOC+, X(HOC+)
along the four sightlines where both N(H2) and N(OH) have
been measured (Liszt & Lucas 2002), sets the relative abundance
scale for HOC+, X(HOC+)
![]() .
.
Note that the OH column density toward
B2200+420 is only 20% larger than that seen along the
archetypical diffuse line of sight toward ![]() Oph,
N(OH) =
Oph,
N(OH) =
![]() (Roueff 1996; Van Dishoeck & Black 1986),
while the reddening (EB-V = 0.33 mag toward B2200+420 from Schlegel et al. (1998)) is
the same. The larger total OH column density seen toward B0355+508
at low galactic latitude (-1.6
(Roueff 1996; Van Dishoeck & Black 1986),
while the reddening (EB-V = 0.33 mag toward B2200+420 from Schlegel et al. (1998)) is
the same. The larger total OH column density seen toward B0355+508
at low galactic latitude (-1.6![]() )
is roughly evenly divided among
5 kinematically-separated diffuse clouds of modest OH and
HCO+ column density, only two of which have prominent CO
emission (Liszt & Lucas 1996; Lucas & Liszt 1996). The line of sight toward
B0415+379 is somewhat darker (see the discussion in
Lucas & Liszt (1998)) but relatively little of the carbon along
even this line of sight is in CO and the density is fairly low,
as gauged by the weakness of emission from species beside CO
(Lucas & Liszt 1996).
)
is roughly evenly divided among
5 kinematically-separated diffuse clouds of modest OH and
HCO+ column density, only two of which have prominent CO
emission (Liszt & Lucas 1996; Lucas & Liszt 1996). The line of sight toward
B0415+379 is somewhat darker (see the discussion in
Lucas & Liszt (1998)) but relatively little of the carbon along
even this line of sight is in CO and the density is fairly low,
as gauged by the weakness of emission from species beside CO
(Lucas & Liszt 1996).
![\begin{figure}
\par\includegraphics[height=9.6cm,width=8.2cm,clip]{1649fig1.eps}\end{figure}](/articles/aa/full/2004/46/aa1649/Timg26.gif) |
Figure 1: HOC+ and H13CO+observed toward B0415+379 (aka 3C 111). |
| Open with DEXTER | |
Table 3: Column density ratios1.
The most obvious path to HOC+ in diffuse gas is via the reaction
![]() ,
which proceeds
with a rate constant
,
which proceeds
with a rate constant
![]() cm3 s-1 in the
UMIST reaction database
cm3 s-1 in the
UMIST reaction database![]() :
these two reactants also produce HCO+ at half the rate,
but this is not an important source of HCO+.
We parametrize the unknown abundance of water relative to OH
and assume that all free carbon is once ionized in a fully
molecular gas having a free carbon abundance
N(C)/N(H) =
:
these two reactants also produce HCO+ at half the rate,
but this is not an important source of HCO+.
We parametrize the unknown abundance of water relative to OH
and assume that all free carbon is once ionized in a fully
molecular gas having a free carbon abundance
N(C)/N(H) =
![]() (Savage & Sembach 1996).
In these terms, the volume formation rate of HOC+ from the reaction
of C+ and water can be expressed as dn(HOC+)/dt=
(Savage & Sembach 1996).
In these terms, the volume formation rate of HOC+ from the reaction
of C+ and water can be expressed as dn(HOC+)/dt=
![]() n(H2)2 X(H2O)/X(OH) cm-3 s-1.
n(H2)2 X(H2O)/X(OH) cm-3 s-1.
![\begin{figure}
\par\includegraphics[height=9.5cm,width=8.05cm,clip]{1649fig2.eps}\end{figure}](/articles/aa/full/2004/46/aa1649/Timg36.gif) |
Figure 2: HOC+ and HCO+ observed toward B2200+420 (aka BL Lac). |
| Open with DEXTER | |
Both HCO+ and HOC+ are formed from the reaction of
H
![]() .
The HCO+/HOC+ branching ratio is typically taken
as 20 (Illies et al. 1982) or more (the current UMIST reaction
database has a branching ratio of 62). The volume formation rate
from this reaction may be written as dn(HOC+)/dt =
.
The HCO+/HOC+ branching ratio is typically taken
as 20 (Illies et al. 1982) or more (the current UMIST reaction
database has a branching ratio of 62). The volume formation rate
from this reaction may be written as dn(HOC+)/dt =
![]() (H2)2(X(H3+) X(CO)/10-12) cm-3 s-1.
The relative abundance of CO is known along these lines of sight
to be 1-5% of the free carbon abundance (Liszt & Lucas 1998), i.e.
(H2)2(X(H3+) X(CO)/10-12) cm-3 s-1.
The relative abundance of CO is known along these lines of sight
to be 1-5% of the free carbon abundance (Liszt & Lucas 1998), i.e.
![]() .
The observed amounts of H3+, while
surprisingly large (McCall et al. 2003) nevertheless still yield
X(H3+) < 10-6.
Thus the only circumstance in which the reaction of H3+ and CO
could be competitive in forming HOC+ would be for very small
water abundances, in which case neither formation route would
suffice to form the observed HOC+.
.
The observed amounts of H3+, while
surprisingly large (McCall et al. 2003) nevertheless still yield
X(H3+) < 10-6.
Thus the only circumstance in which the reaction of H3+ and CO
could be competitive in forming HOC+ would be for very small
water abundances, in which case neither formation route would
suffice to form the observed HOC+.
HOC+ is likely destroyed by two processes which proceed with
very nearly equal rates in diffuse gas. The HOC+ isomer lies
some 17 000 K higher in energy compared to
HCO+ and interconversion by molecular hydrogen
(HOC+ + H2
![]() HOC+ + H2) has recently been
shown to proceed with a rate
constant
HOC+ + H2) has recently been
shown to proceed with a rate
constant
![]() s-1nearly independent of
temperature between 25 K and 300 K (Smith et al. 2002).
This is 70% smaller than in the UMIST database, enhancing
the prospects for reproducing X(HOC+).
The destruction rate for isomerization can be written
as dn(HOC+)/dt =
s-1nearly independent of
temperature between 25 K and 300 K (Smith et al. 2002).
This is 70% smaller than in the UMIST database, enhancing
the prospects for reproducing X(HOC+).
The destruction rate for isomerization can be written
as dn(HOC+)/dt =
![]() n(HOC+) cm-3 s-1.
n(HOC+) cm-3 s-1.
Dissociative recombination,
![]() ,
proceeds
with a rate constant
,
proceeds
with a rate constant
![]() cm 3 s-1.
Assuming that free carbon is once-ionized and expressing the
electron fraction in terms of the ratio of free carbon to
cm 3 s-1.
Assuming that free carbon is once-ionized and expressing the
electron fraction in terms of the ratio of free carbon to
![]() expected for a fully molecular diffuse gas (i.e.
N(C)/N(H2) =
expected for a fully molecular diffuse gas (i.e.
N(C)/N(H2) =
![]() (Savage & Sembach 1996)).
The destruction rate from recombination can be written as
dn(HOC+)/d
(Savage & Sembach 1996)).
The destruction rate from recombination can be written as
dn(HOC+)/d
![]() (X(e)/X(C+)) cm-3 s-1.
The expected electron abundance is a small multiple of the
free carbon abundance (Liszt 2003) so dissociative recombination
and interconversion probably compete quite effectively to destroy
HOC+.
(X(e)/X(C+)) cm-3 s-1.
The expected electron abundance is a small multiple of the
free carbon abundance (Liszt 2003) so dissociative recombination
and interconversion probably compete quite effectively to destroy
HOC+.
The net destruction rate from both processes is of order
-10-9 n(H2) cm-3 s-1 and the expected abundance of
HOC+ is
X(HOC+)
![]() X(H2O)/X(OH) or
X(HOC+)/X(OH)
X(H2O)/X(OH) or
X(HOC+)/X(OH)
![]() X(H2O)/X(OH).
The observed amounts of HOC+ can be made in diffuse
gas if the abundance of water is comparable to that of
OH. Limits on the OH/water ratio in diffuse clouds
N(OH)/N(H2O) > 2-3 were derived by Van Dishoeck & Black (1986) toward
X(H2O)/X(OH).
The observed amounts of HOC+ can be made in diffuse
gas if the abundance of water is comparable to that of
OH. Limits on the OH/water ratio in diffuse clouds
N(OH)/N(H2O) > 2-3 were derived by Van Dishoeck & Black (1986) toward
![]() Oph and
Oph and ![]() Per.
Per.
Given the weakness of the limits on the water abundance in diffuse
clouds, straightforward gas-phase chemistry fails only weakly to
produce the observed amount of HOC+. This situation can be
compared to the case of HCO+ where the thermal reaction of
C+ upon the known quantities of OH falls 30-50 times short
of producing the observed values of N(HCO+) via the reaction
chain
C
![]() .
At thermal rates, this series
of reactions (which predicts an HCO+/OH ratio dependent only on
temperature) produces N(HCO+) scarcely larger than the observed
N(HOC+).
The predicted abundance of HCO+ could be increased in quiescent gas
by hypothesizing that it recombines slowly, but only at the cost
of understanding CO (Liszt & Lucas 2000).
.
At thermal rates, this series
of reactions (which predicts an HCO+/OH ratio dependent only on
temperature) produces N(HCO+) scarcely larger than the observed
N(HOC+).
The predicted abundance of HCO+ could be increased in quiescent gas
by hypothesizing that it recombines slowly, but only at the cost
of understanding CO (Liszt & Lucas 2000).
Clearly, knowledge of the abundance of water is the limiting factor in assessment of the diffuse cloud chemistry of HOC+.
The work presented here is the first evidence of HOC+ in diffuse
gas, but even the case for its existence in the dense
molecular ISM was marginal before the pioneering work of Apponi
and his collaborators during a brief period in the mid-1990's while
the NRAO 12 m telescope was still operating.
To summarize the prior results we note that the
HCO+/HOC+ ratio in dense dark gas is
typically as large as 1000-9000 (Apponi & Ziurys 1997; Ziurys & Apponi 1995), but is
found to be much smaller (50-400) in dense PDR like the Orion Bar
(Apponi et al. 1999) and/or NGC 7023 (Fuente et al. 2003). In these PDR
the relative abundance of HOC+ is X(HOC+)
![]() ,
quite similar to the diffuse clouds studied here.
,
quite similar to the diffuse clouds studied here.
The difference between the HCO+/HOC+ ratios seen in
diffuse and dark dense gas is largely the result
of the low ionization fraction in the latter. Whereas HOC+ is destroyed only slightly faster than HCO+ in diffuse gas,
the isomerization reaction undergone by HOC+ is much faster than
recombination when the electron fraction is 10-7 instead of
10-4. In dense dark gas HCO+ and HCO+ are formed by
H
![]() with
a branching ratio 20-60:1 in favor of HCO+ and this ratio is
increased by another
factor of 100 by the unequal destruction rates.
Under favorable conditions, measured HCO+/HOC+ ratios could
be good diagnostics of the ionization fraction in dense gas.
with
a branching ratio 20-60:1 in favor of HCO+ and this ratio is
increased by another
factor of 100 by the unequal destruction rates.
Under favorable conditions, measured HCO+/HOC+ ratios could
be good diagnostics of the ionization fraction in dense gas.
Reasoning by analogy from the discussion of diffuse and dark gas, we infer that the relatively low HCO+/HOC+ ratios in dense PDR also reflect the relative weakness of isomerization. In cases where isomerization is weak both isomers are destroyed by recombination at roughly equal rates, and their abundance ratio is more directly indicative of the relative rates at which the two isomers are formed.
We have added HOC+ to the roster of polyatomic molecules known to
exist in diffuse clouds. The HCO+/HOC+ ratio, 70-120, is much
smaller than in dense dark clouds but quite comparable to the most
extreme values seen in dense PDR. The inferred relative abundance
X(HOC+)
![]() ,
similar to that seen in dense
PDR, can be synthesized at thermal rates
in quiescent diffuse gas if the water/OH ratio is of order unity.
This is a mild discrepancy, much less severe than is inferred
for HCO+, as the water/OH ratio is currently known only to be less
than 0.3-0.5.
,
similar to that seen in dense
PDR, can be synthesized at thermal rates
in quiescent diffuse gas if the water/OH ratio is of order unity.
This is a mild discrepancy, much less severe than is inferred
for HCO+, as the water/OH ratio is currently known only to be less
than 0.3-0.5.
Several authors have recently noted that hydrocarbons like 3h2 and C2H have unexpectedly high abundances in dense PDR (Fuente et al. 2003; Fosse et al. 2002; Teyssier et al. 2004), very much as has been observed in diffuse clouds (Lucas & Liszt 2000). The HCO+/HOC+ ratios found here in diffuse clouds, which are PDR of lower density and presumably weaker illumination (hence similar ionization rates per H2), constitute another example of chemical similarities
between dense and diffuse PDR. It will be interesting to see how much more complete a parallel can be drawn between the two regimes.
Acknowledgements
The National Radio Astronomy Observatory is operated by AUI, Inc. under a cooperative agreement with the US National Science Foundation. IRAM is operated by CNRS (France), the MPG (Germany) and the IGN (Spain). We owe the staff the Plateau de Bure our thanks for their assistance in taking the data. We thank the referee for helpful comments.