A&A 412, 133-145 (2003)
DOI: 10.1051/0004-6361:20031409
Sulphur chemistry in the envelopes of massive young stars
F. F. S. van der Tak 1 - A. M. S. Boonman 2 -
R. Braakman 2 - E. F. van Dishoeck2
1 - Max-Planck-Institut für Radioastronomie, Auf dem Hügel
69, 53121 Bonn, Germany
2 - Sterrewacht, Postbus 9513, 2300 RA
Leiden, The Netherlands
Received 9 December 2002 / Accepted 20 August 2003
Abstract
The sulphur chemistry in nine regions in the earliest
stages of high-mass star formation is studied through single-dish
submillimeter spectroscopy. The line profiles indicate that
10-50% of the SO and SO2 emission arises in high-velocity gas,
either infalling or outflowing. For the low-velocity gas, excitation
temperatures are 25 K for H2S, 50 K for SO, H2CS, NS and HCS+,
and 100 K for OCS and SO2, indicating that most observed emission
traces the outer parts (T<100 K) of the molecular envelopes,
except high-excitation OCS and SO2 lines. Abundances in the outer
envelopes, calculated with a Monte Carlo program, using the physical
structures of the sources derived from previous submillimeter
continuum and CS line data, are  10-8 for OCS,
10-8 for OCS,
 10-9 for H2S, H2CS, SO and SO2, and
10-9 for H2S, H2CS, SO and SO2, and  10-10 for HCS+ and NS. In the inner envelopes (T>100 K) of six
sources, the SO2 abundance is enhanced by a factor of
10-10 for HCS+ and NS. In the inner envelopes (T>100 K) of six
sources, the SO2 abundance is enhanced by a factor of
 100-1000. This region of hot, abundant SO2 has been seen
before in infrared absorption, and must be small,
100-1000. This region of hot, abundant SO2 has been seen
before in infrared absorption, and must be small,  0
0
 2
(180 AU radius). The derived abundance profiles are consistent with
models of envelope chemistry which invoke ice evaporation at
2
(180 AU radius). The derived abundance profiles are consistent with
models of envelope chemistry which invoke ice evaporation at  K. Shock chemistry is unlikely to contribute. A major sulphur
carrier in the ices is probably OCS, not H2S as most models
assume. The source-to-source abundance variations of most molecules
by factors of
K. Shock chemistry is unlikely to contribute. A major sulphur
carrier in the ices is probably OCS, not H2S as most models
assume. The source-to-source abundance variations of most molecules
by factors of  10 do not correlate with previous systematic
tracers of envelope heating. Without observations of H2S and SO
lines probing warm (
10 do not correlate with previous systematic
tracers of envelope heating. Without observations of H2S and SO
lines probing warm ( 100 K) gas, sulphur-bearing molecules
cannot be used as evolutionary tracers during star formation.
100 K) gas, sulphur-bearing molecules
cannot be used as evolutionary tracers during star formation.
Key words: ISM: molecules - molecular processes - stars:
circumstellar matter - stars: formation
1 Introduction
Spectral line surveys at submillimeter wavelengths have revealed
considerable chemical differences between star-forming regions (e.g.,
Blake et al. 1987; Nummelin et al. 2000; Helmich & van Dishoeck 1997; Schilke et al. 1997; Hatchell et al. 1998a; Sutton et al. 1995; see
van Dishoeck 2001 for a complete list). While these differences indicate
activity, the dependence of molecular abundances on evolutionary state
and physical parameters is poorly understood. Better insight into this
relation would be valuable for probing the earliest, deeply embedded
phases of star formation where diagnostics at optical and
near-infrared wavelengths are unavailable. This is especially true for
the formation of high-mass stars, for which the order in which
phenomena occur is much less well understood than in the low-mass
case, and for which the embedded phase is a significant fraction
( 10%) of the total lifetime of
10%) of the total lifetime of  106 yr.
106 yr.
Sulphur-bearing molecules are attractive as candidate tracers of early
protostellar evolution (Buckle & Fuller 2003; Hatchell et al. 1998b). In models of "hot
cores'' (Charnley 1997), where the chemistry is driven by the
evaporation of icy grain mantles due to protostellar heating, the
abundances of sulphur-bearing molecules exhibit a characteristic
variation with time. After its release from the grain mantles, H2S
is converted into SO and SO2 in  103 yr, and further into
CS, H2CS and OCS after
103 yr, and further into
CS, H2CS and OCS after  105 yr. This type of model has had
some success in reproducing the chemistry of star-forming regions
(Langer et al. 2000).
105 yr. This type of model has had
some success in reproducing the chemistry of star-forming regions
(Langer et al. 2000).
Besides thermal heating, shocks are potentially relevant to the
chemistry in star-forming regions. Young stars drive outflows which
expose their surroundings to the effects of shocks. The submillimeter
lines of SO2 in Orion are very broad, and likely arise in the
outflow (Schilke et al. 1997). Models of chemistry behind interstellar
shocks (Pineau des Forêts et al. 1993; Mitchell 1984; Leen & Graff 1988) predict enhancements of H2S, SO
and SO2 on time scales of  104 yr. Thus, both by ice
evaporation and by shock interaction, sulphur could act as a clock on
the time scale of 104 yr relevant for the embedded phase of star
formation.
104 yr. Thus, both by ice
evaporation and by shock interaction, sulphur could act as a clock on
the time scale of 104 yr relevant for the embedded phase of star
formation.
![\begin{figure}
\par\resizebox{16.2cm}{!}{\includegraphics[angle=-90,clip]{ms3386.f1}} \end{figure}](/articles/aa/full/2003/47/aa3386/Timg11.gif) |
Figure 1:
Two examples of spectral settings toward eight of our
sources, showing differences in the relative and in the absolute
strengths of lines of sulphur-bearing molecules. The top and
bottom frequency scales are the two receiver sidebands. The data
for NGC 6334 IRS1 have been divided by 10 and 3 respectively,
for clarity. The non-labeled lines are due to
non-sulphur-bearing molecules, of which the strongest are
labeled in the AFGL 2591 panels. |
| Open with DEXTER |
This paper uses submillimeter observations of sulphur-bearing
molecules to test models of chemistry during star formation, and
attempts to order these regions chronologically. The sources are nine
regions of high-mass star formation with luminosities

 at distances of 1-4 kpc (Table 1).
Originally selected for their mid-infrared brightness, the sources
were found to have large envelope masses (40-1100
at distances of 1-4 kpc (Table 1).
Originally selected for their mid-infrared brightness, the sources
were found to have large envelope masses (40-1100  within
r=0.15-0.36 pc) based on submillimeter data (van der Tak et al. 2000b). Together
with their weak radio emission and their strong molecular outflows,
these masses indicate very early evolutionary stages, probably
preceding the formation of hot cores. Two of our sources may be more
evolved than the others: NGC 6334 IRS1, which has a very rich, "hot
core''-type submillimeter spectrum (McCutcheon et al. 2000; Thorwirth et
al., in prep.), and NGC 7538 IRS1, which has strong free-free
emission.
within
r=0.15-0.36 pc) based on submillimeter data (van der Tak et al. 2000b). Together
with their weak radio emission and their strong molecular outflows,
these masses indicate very early evolutionary stages, probably
preceding the formation of hot cores. Two of our sources may be more
evolved than the others: NGC 6334 IRS1, which has a very rich, "hot
core''-type submillimeter spectrum (McCutcheon et al. 2000; Thorwirth et
al., in prep.), and NGC 7538 IRS1, which has strong free-free
emission.
Observations of CH3OH lines towards some of these sources
(van der Tak et al. 2000a) indicate an increase in the CH3OH abundance by a
factor of 100 in the warm inner envelope, which is likely due to
evaporation of CH3OH-rich ices. The excitation of CH3OH, as well
as that of CO and C2H2, correlates well with other temperature
tracers, such as the gas/solid ratios of CO2 and H2O, the
45/100  m colour and the fraction of heated solid 13CO2
(Boogert et al. 2000; Boonman et al. 2003; van der Tak et al. 2000b). Since these quantities also
correlate with the ratio of envelope mass to stellar mass, their
variation likely reflects evolution through the dispersal of the
protostellar envelopes. These findings make the sample a good testbed
for theories of chemical evolution during the earliest deeply embedded
phases of high-mass star formation.
m colour and the fraction of heated solid 13CO2
(Boogert et al. 2000; Boonman et al. 2003; van der Tak et al. 2000b). Since these quantities also
correlate with the ratio of envelope mass to stellar mass, their
variation likely reflects evolution through the dispersal of the
protostellar envelopes. These findings make the sample a good testbed
for theories of chemical evolution during the earliest deeply embedded
phases of high-mass star formation.
Table 1:
Source samplea.
2 Observations
The data presented in this paper are part of a targeted
spectral line survey of embedded massive stars, aimed to track
chemical evolution during the earliest stages of high-mass star
formation. Fifteen frequency settings have been observed, which also
contain many lines of sulphurless molecules. Some of these data have
been published by van der Tak et al. (1999,2000b,a). These observations
were performed with the 15-m James Clerk Maxwell Telescope
(JCMT)![[*]](/icons/foot_motif.gif) on Mauna Kea, Hawaii between 1995 and 1998. The beam size
(FWHM) and main beam efficiency of the antenna were 18'' and
64-69% at 230 GHz and 14'' and 58-63% at 345 GHz.
The frontends were the receivers A2, B3 and B3i; the backend was the
Digital Autocorrelation Spectrometer, covering 500 MHz instantaneous
bandwidth. Pointing was checked every 2 hours during the observing and
was always found to be within 5''. To subtract the atmospheric and
instrumental background, the chopping secondary was used with 180''offsets. Total integration times are 30-40 min for each frequency
setting. Figure 1 shows examples of the data towards all
but one of our sources; the data on W3 IRS5 were presented by
Helmich & van Dishoeck (1997).
on Mauna Kea, Hawaii between 1995 and 1998. The beam size
(FWHM) and main beam efficiency of the antenna were 18'' and
64-69% at 230 GHz and 14'' and 58-63% at 345 GHz.
The frontends were the receivers A2, B3 and B3i; the backend was the
Digital Autocorrelation Spectrometer, covering 500 MHz instantaneous
bandwidth. Pointing was checked every 2 hours during the observing and
was always found to be within 5''. To subtract the atmospheric and
instrumental background, the chopping secondary was used with 180''offsets. Total integration times are 30-40 min for each frequency
setting. Figure 1 shows examples of the data towards all
but one of our sources; the data on W3 IRS5 were presented by
Helmich & van Dishoeck (1997).
Additional observations of H2S and OCS in the 130-180 GHz window
were carried out with the 30-m telescope of the Institut de Radio
Astronomie Millimétrique (IRAM)![[*]](/icons/foot_motif.gif) on Pico Veleta, Spain, in August 2002. The frontend was
the facility receiver C150 and the backend the Versatile Spectral
Assembly (VESPA) autocorrelator. Integration times were 10-20 min
using frequency switching with a throw of 7.3 MHz. Data were
calibrated onto
on Pico Veleta, Spain, in August 2002. The frontend was
the facility receiver C150 and the backend the Versatile Spectral
Assembly (VESPA) autocorrelator. Integration times were 10-20 min
using frequency switching with a throw of 7.3 MHz. Data were
calibrated onto
 scale by multiplying by 1.43, the ratio of
forward and main beam efficiencies. The beam size is 15'' at these
frequencies.
scale by multiplying by 1.43, the ratio of
forward and main beam efficiencies. The beam size is 15'' at these
frequencies.
The H2S 393 GHz line was observed in May 1995 at the 10.4-m Caltech
Submillimeter Observatory (CSO)![[*]](/icons/foot_motif.gif) , with the facility receiver as frontend and the
50 MHz AOS as backend. The telescope has a beam size of 25'' and a
main beam efficiency of 60% at this frequency.
, with the facility receiver as frontend and the
50 MHz AOS as backend. The telescope has a beam size of 25'' and a
main beam efficiency of 60% at this frequency.
Table 2:
Observed transitions.
Reduction was carried out using the CLASS package developed at IRAM.
Linear baselines were subtracted and the spectra were smoothed once
and calibrated onto
 scale. Only the frequency switched 30 m data
required higher order polynomials to fit the baseline. The final
spectra have a resolution of 0.3-1.5 km s-1 and rms noise levels (in
scale. Only the frequency switched 30 m data
required higher order polynomials to fit the baseline. The final
spectra have a resolution of 0.3-1.5 km s-1 and rms noise levels (in
 )
of 20-30 mK for the 150 and 230 GHz bands, and 30-50 mK for
the 345 GHz band. Multiplying these values by the line width
(Table 4) yields the uncertainties of the line fluxes in
Table 3. Although the absolute calibration is only
correct to
)
of 20-30 mK for the 150 and 230 GHz bands, and 30-50 mK for
the 345 GHz band. Multiplying these values by the line width
(Table 4) yields the uncertainties of the line fluxes in
Table 3. Although the absolute calibration is only
correct to  30%, the relative strength of lines within one
frequency setting is much more accurate.
30%, the relative strength of lines within one
frequency setting is much more accurate.
Line identification was performed using the JPL catalog
(Pickett et al. 1998)![[*]](/icons/foot_motif.gif) . A matching
frequency is the main criterion for assignment, but the upper energy
level of the transition and the complexity of the molecule are also
considered. Table 2 has details about the observed
transitions.
. A matching
frequency is the main criterion for assignment, but the upper energy
level of the transition and the complexity of the molecule are also
considered. Table 2 has details about the observed
transitions.
For each detected feature, a satisfying match could be found in the
catalog within 0.5 MHz, except for the H2CS
1019-918 line at
348.5 GHz, whose residual is 2 MHz. Laboratory spectroscopy of H2CS
only exists up to 250 GHz (Beers et al. 1972), and the analysis in the JPL
catalog only includes pure rotation and centrifugal distortion terms.
At higher frequencies and for higher J-values, higher order terms
become important, which may be why the catalog prediction is
inaccurate for this line. Our data indicate a frequency of
348 534.2 MHz.
3 Results
3.1 Line profiles
We have fitted the detected lines with Gaussian profiles.
Table 3 reports the observed fluxes and
Table 4 the widths of the lines. While most lines could
be satisfactorily fitted with a single Gaussian, the lines of SO,
which are the strongest lines that we have observed, required two
Gaussians for a good fit to their profiles (Fig. 2;
Table 4). One component agrees in position and width
with the values measured for C17O and C34S by van der Tak et al. (2000b)
and is attributed to a static envelope. Table 5
reports similar measurements for the source MonR2 IRS3 which van der Tak et al. did not study. The other component has 2.8 times as
large a width on average. This high-velocity emission may trace
outflowing or infalling motions. The contributions from high- and
low-velocity gas to the SO line fluxes are about equal, unlike CS,
where the envelope carries 90% of the fluxes (van der Tak et al. 2000b;
Table 5). The infrared absorption and submillimeter
emission lines of CO towards these sources also show multiple velocity
components (Mitchell et al. 1992; Mitchell & Hasegawa 1991; Mitchell et al. 1991). However, the
velocities do not match in detail, as expected because of differences
in spectral and angular resolution between infrared and submillimeter
data on one hand, and excitation differences between CO and SO on the
other.
The fit of two Gaussians to the SO lines is excellent, which is not
always the case for CO. An alternative method is to use area
integrals, which do not attribute any low-velocity gas to the
high-velocity component. Our approach may overestimate the
high-velocity contribution to the line flux by factors up to 2.
The ratios of the high- to low-velocity contributions to the SO lines
are  1 for the lines in the 230 GHz band, and
1 for the lines in the 230 GHz band, and  1 for
the lines in the 345 GHz band. This difference could be due to the
higher temperatures and densities required for excitation of the
345 GHz lines, or to the smaller beams in which they are observed.
Since the high-velocity wings on the CS lines in these sources also
become more pronounced in smaller beams, independent of transition
(van der Tak et al. 2000b), the broad component probably is more compact than the
envelope, but not necessarily warmer or denser. The high-velocity gas
in these sources thus seems to be confined to small radii. This
situation differs from that in molecular clouds, where line width
increases with size, and from that in many molecular outflows, where
velocities usually increase with radius (e.g., Lee et al. 2002).
However, while CO data show arcminute-sized outflows in our sources,
higher velocities naturally occur at smaller radii in the case of
infall. For example, gas at 1000 AU (1'' at 1 kpc) in free fall
onto a 10
1 for
the lines in the 345 GHz band. This difference could be due to the
higher temperatures and densities required for excitation of the
345 GHz lines, or to the smaller beams in which they are observed.
Since the high-velocity wings on the CS lines in these sources also
become more pronounced in smaller beams, independent of transition
(van der Tak et al. 2000b), the broad component probably is more compact than the
envelope, but not necessarily warmer or denser. The high-velocity gas
in these sources thus seems to be confined to small radii. This
situation differs from that in molecular clouds, where line width
increases with size, and from that in many molecular outflows, where
velocities usually increase with radius (e.g., Lee et al. 2002).
However, while CO data show arcminute-sized outflows in our sources,
higher velocities naturally occur at smaller radii in the case of
infall. For example, gas at 1000 AU (1'' at 1 kpc) in free fall
onto a 10  object would have
object would have
 km s-1, consistent
with the observed width of the broad component (Table 4).
km s-1, consistent
with the observed width of the broad component (Table 4).
The broad component peaks at slightly more blueshifted velocities (by
0.17 km s-1 on average) than the envelope, a situation found before
for CS and H2CO (van der Tak et al. 2000b). In the case of outflow, obscuration
by AV=104 mag of dust could explain the absence of redshifted
wings. Since some redshifted SO is seen (Fig. 2), the
requirement relaxes to several 1000 mag. Of the sources discussed
here, only NGC 6334 IRS1 may have such a large amount of dust in its
envelope (Table 1); the other sources may need
circumstellar disks seen face-on. In the case of infall, a mixture of
absorption and emission is expected at redshifted velocities
(Choi 2002), which may partly cancel each other if unresolved, to
give the impression of predominantly blueshifted emission. We
conclude that both infall and outflow are plausible explanations for
the high-velocity gas, and recommend interferometric observations of
SO to decide between these options.
![\begin{figure}
\par\resizebox{8.8cm}{!}{\includegraphics[angle=-90,clip]{ms3386.f2}} \end{figure}](/articles/aa/full/2003/47/aa3386/Timg81.gif) |
Figure 2:
Observed velocity profiles of the SO 66-55 and
87-76 lines in the sources AFGL 2591 and W3 IRS5,
showing the low- and high-velocity components. The dashed lines
are our fits to the individual components and the dotted line is
the sum of the two components. |
| Open with DEXTER |
For most sources in our sample, the width of the SO2 lines is
between the values for the low- and high-velocity components. The
only source where the SO2 lines are strong enough to fit two
components is W3 IRS5, where the line profiles do not show wings.
However, most of these data, taken from Helmich & van Dishoeck (1997), were observed
with an AOS as backend, and have lower spectral resolution than our
data. It seems therefore plausible that, like for SO, about half of
the SO2 emission arises in high-velocity gas.
Table 3:
Observed line fluxes
 (K km s-1)
from Gaussian fits, and 1
(K km s-1)
from Gaussian fits, and 1 upper limitsa.
upper limitsa.
Table 4:
Widths  (km s-1) of the emission line components,
averaged over transition. Numbers in brackets denote uncertainties
in units of the last decimal, except when only one transition was
observed. Dots denote missing data. For SO, values for the narrow
and the broad components are
reported, whose positions are also given.
(km s-1) of the emission line components,
averaged over transition. Numbers in brackets denote uncertainties
in units of the last decimal, except when only one transition was
observed. Dots denote missing data. For SO, values for the narrow
and the broad components are
reported, whose positions are also given.
For the other molecules, the signal to noise ratio is not high enough
to disentangle multiple velocity components. This paper assumes that
these lines originate in low-velocity gas, which given our results for
SO may overestimate column densities and abundances
(Sects. 3.3, 4) by factors of up to 2.
Table 5:
Observations of CS, C34S and C17O lines towards MonR2 IRS3.
3.2 Excitation temperatures
For SO and SO2, the number of detected lines is large enough to
construct rotation diagrams. This method, described in detail by
Blake et al. (1987) and Helmich et al. (1994), assumes that the lines are
optically thin and that the molecular excitation can be described by a
single temperature, the rotation temperature. If radiative decay
competes with collisional excitation, the rotation temperature
lies below the kinetic temperature. If the lines are optically thick,
the rotation diagram underestimates the column density and
overestimates the excitation temperature. Another complication is that
the lines are measured in beams of unequal size, so that somewhat
different volumes of gas are probed. Despite these caveats, the
rotation diagram provides a useful first estimate of excitation
conditions and molecular column densities, and a stepping stone
towards a more sophisticated analysis (Sect. 4).
Table 6:
Rotation temperatures (K). Numbers in brackets denote uncertainties.
Table 6 presents the results. In the case of SO, separate
fits were made to the low- and high-velocity components. For NGC 7538 IRS9, the detected SO2 lines do not cover a large enough range of
energy levels to constrain
 .
The derived temperatures are
30...70 K for SO and 50...190 K for SO2, reflecting the available
range of energy levels. Lower limits to
.
The derived temperatures are
30...70 K for SO and 50...190 K for SO2, reflecting the available
range of energy levels. Lower limits to
 are poor fits to the
data, probably caused by optically thick lines. Indeed, the
SO/34SO and SO2/34SO2 line ratios of 1.3...
are poor fits to the
data, probably caused by optically thick lines. Indeed, the
SO/34SO and SO2/34SO2 line ratios of 1.3... 10
are below the isotopic abundance ratio of 32S/
10
are below the isotopic abundance ratio of 32S/
 ,
which
indicates optical depths of a few in the SO and SO2 lines.
,
which
indicates optical depths of a few in the SO and SO2 lines.
Table 7:
Column densities (cm-2) in 15-20'' beams.
![\begin{figure}
\par\resizebox{8.5cm}{!}{\includegraphics[clip]{ms3386.f3}} \end{figure}](/articles/aa/full/2003/47/aa3386/Timg139.gif) |
Figure 3:
Rotation temperatures of SO (dots) and SO2 (triangles)
versus values for CH3OH. |
| Open with DEXTER |
The last column of Table 6 lists rotation temperatures of
CH3OH, measured previously. Strictly, these numbers are only lower
limits to the kinetic temperatures in a 15'' beam, but they show
good correlation with
 (C2H2) which does directly trace the
kinetic temperature (van der Tak et al. 2000a). The rotation temperatures of SO
and SO2 are not clearly correlated with
(C2H2) which does directly trace the
kinetic temperature (van der Tak et al. 2000a). The rotation temperatures of SO
and SO2 are not clearly correlated with
 (CH3OH) within the
uncertainties (Fig. 3). Therefore we cannot conclude at
this point whether these molecules trace the same gas within the
envelopes. In any case, differences in beam sizes and optical depth
effects in the lines make it hard to draw firm conclusions before a
more detailed radiative transfer analysis is presented in
Sect. 4.
(CH3OH) within the
uncertainties (Fig. 3). Therefore we cannot conclude at
this point whether these molecules trace the same gas within the
envelopes. In any case, differences in beam sizes and optical depth
effects in the lines make it hard to draw firm conclusions before a
more detailed radiative transfer analysis is presented in
Sect. 4.
For the other molecules, for which only few lines are available, the
line ratios have been used to constrain
 .
Source-averaged line
ratios indicate
.
Source-averaged line
ratios indicate

 50 K for H2CS, NS and HCS+,
50 K for H2CS, NS and HCS+,

 25 K for H2S and
25 K for H2S and

 100 K for
OCS. These values are only rough estimates, especially if
based on few sources and/or lines.
100 K for
OCS. These values are only rough estimates, especially if
based on few sources and/or lines.
3.3 Column densities
Table 7 presents beam-averaged molecular column
densities, estimated from the observed line fluxes using the
excitation temperatures found above. The column densities for H2CS
and H2S are the sums of the ortho and para species, assuming an
ortho to para ratio of 3. After calibration, the adopted
 is the
main source of uncertainty in the column densities, especially if only
one or two lines have been observed. For example, decreasing
is the
main source of uncertainty in the column densities, especially if only
one or two lines have been observed. For example, decreasing
 from 50 to 25 K increases column density estimates by factors of
from 50 to 25 K increases column density estimates by factors of
 2, while increasing
2, while increasing
 from 50 to 75 K decreases column
density estimates by
from 50 to 75 K decreases column
density estimates by  20%. In addition, column densities may
have been underestimated by factors of a few due to nonnegligible
optical depth.
20%. In addition, column densities may
have been underestimated by factors of a few due to nonnegligible
optical depth.
To search for trends of column densities with temperature,
Table 7 lists the sources in order of increasing
T(CH3OH). The only trend seems to be that the
warmest source has the largest column density of all molecules, except
SO and SO2. In order to investigate whether these trends in the
column density reflect chemical differences between sources or are due
to optical depth or beam size effects, a more detailed radiative
transfer analysis is presented in Sect. 4.
3.4 Comparison with infrared data
Keane et al. (2001) observed the  band of SO2 around 7.3
band of SO2 around 7.3  m in
absorption towards five of the sources studied here, and found column
densities of SO2 of a few 1016 cm-2, a factor of
m in
absorption towards five of the sources studied here, and found column
densities of SO2 of a few 1016 cm-2, a factor of  100
higher than the submillimeter data indicate. In contrast, infrared
observations of CO and dust yield 3-5 times lower column
densities than submillimeter data, as a result of non-spherical
geometry on scales
100
higher than the submillimeter data indicate. In contrast, infrared
observations of CO and dust yield 3-5 times lower column
densities than submillimeter data, as a result of non-spherical
geometry on scales 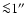 (van der Tak et al. 2000b). Correcting for
optical depth in the SO2 submillimeter lines increases the column
density estimate, but only by factors of a few (Sect. 3.2),
which does not explain the discrepancy with the infrared values. More
likely to be important are chemical effects on small scales. The
sources are centrally concentrated, n(H2)
(van der Tak et al. 2000b). Correcting for
optical depth in the SO2 submillimeter lines increases the column
density estimate, but only by factors of a few (Sect. 3.2),
which does not explain the discrepancy with the infrared values. More
likely to be important are chemical effects on small scales. The
sources are centrally concentrated, n(H2)
 with
with
 ,
so that absorption data are more sensitive to warm,
dense gas at small radii, while emission data probe more extended,
cooler and less dense gas. The infrared excitation temperatures of
225-750 K also suggest that the SO2 absorption arises in warm gas.
,
so that absorption data are more sensitive to warm,
dense gas at small radii, while emission data probe more extended,
cooler and less dense gas. The infrared excitation temperatures of
225-750 K also suggest that the SO2 absorption arises in warm gas.
The infrared estimates of N(SO2) imply optically thick
submillimeter lines of SO2, but their brightness measured with the
JCMT is much lower than the infrared
 .
The implied limit on the
source size is
.
The implied limit on the
source size is
 ,
with T0the typical
,
with T0the typical
 of 0.1 K. Inferred values of
of 0.1 K. Inferred values of
 range from
0
range from
0
 17 for AFGL 2591 to 0
17 for AFGL 2591 to 0
 32 for MonR2 IRS3, corresponding to
linear radii of 100-250 AU. The limited spectral resolution of the
infrared data prohibits assignment of the absorbers to either velocity
component in emission, which however does not affect our conclusion.
32 for MonR2 IRS3, corresponding to
linear radii of 100-250 AU. The limited spectral resolution of the
infrared data prohibits assignment of the absorbers to either velocity
component in emission, which however does not affect our conclusion.
4 Radiative transfer analysis
To estimate molecular abundances we have used the Monte Carlo
radiative transfer program by Hogerheijde & van der Tak (2000)![[*]](/icons/foot_motif.gif) .
Starting from radial profiles of
the density and kinetic temperature, this program solves for the
molecular excitation as a function of radius. Besides collisional
excitation, radiation from the cosmic microwave background and thermal
radiation from local dust are taken into account. The result is
integrated over the line of sight and convolved with the appropriate
telescope beam. Observed and synthetic line fluxes are compared with a
.
Starting from radial profiles of
the density and kinetic temperature, this program solves for the
molecular excitation as a function of radius. Besides collisional
excitation, radiation from the cosmic microwave background and thermal
radiation from local dust are taken into account. The result is
integrated over the line of sight and convolved with the appropriate
telescope beam. Observed and synthetic line fluxes are compared with a
 statistic to find the best-fit abundance. Initially,
abundances were assumed to be constant with radius.
statistic to find the best-fit abundance. Initially,
abundances were assumed to be constant with radius.
Molecular data were mostly taken from the database by Schöier et
al. (in prep.)![[*]](/icons/foot_motif.gif) ,
which paper also describes our extension of the SO2 rate
coefficients by Green (1995) to higher energy levels. No rate
coefficients are available for NS, so its excitation was assumed to be
thermalized at the temperature of each grid point of the model. The
population of the
,
which paper also describes our extension of the SO2 rate
coefficients by Green (1995) to higher energy levels. No rate
coefficients are available for NS, so its excitation was assumed to be
thermalized at the temperature of each grid point of the model. The
population of the
 state of NS, which lies 225 K above
the
state of NS, which lies 225 K above
the
 state, was assumed to be negligible. In the case of
H2S, Ball et al. (1999) measured the 110-101 rate coefficient in
collisions with He at
T=1.36-35.3 K. Our model uses the rate
coefficients by Green et al. (1993) for H2O-He, multiplied by 2.6 based
on comparison with the Ball et al. data, and scaled for the different
reduced mass of the H2S-H2 system. Turner (1996) lists reasons
why H2S rate coefficients are likely to be larger than H2O values.
state, was assumed to be negligible. In the case of
H2S, Ball et al. (1999) measured the 110-101 rate coefficient in
collisions with He at
T=1.36-35.3 K. Our model uses the rate
coefficients by Green et al. (1993) for H2O-He, multiplied by 2.6 based
on comparison with the Ball et al. data, and scaled for the different
reduced mass of the H2S-H2 system. Turner (1996) lists reasons
why H2S rate coefficients are likely to be larger than H2O values.
The temperature and density structures of all our sources but MonR2
IRS3 were derived by van der Tak et al. (2000b). We have followed the same
procedure to infer the structure of MonR2 IRS3, assuming a power-law
density structure
 .
The temperature profile is
derived from the observed luminosity of
.
The temperature profile is
derived from the observed luminosity of

 (Henning et al. 1992), while n0 is constrained by the submillimeter
photometry of Giannakopoulou et al. (1997). These dust models solve the grain
heating and cooling self-consistenly, using opacity model 5 from
Ossenkopf & Henning (1994). These "OH5'' opacities are the only ones that yield
dust masses consistent with C17O measurements of AFGL 2591, where
CO depletion is known to be negligible (van der Tak et al. 1999). To constrain
(Henning et al. 1992), while n0 is constrained by the submillimeter
photometry of Giannakopoulou et al. (1997). These dust models solve the grain
heating and cooling self-consistenly, using opacity model 5 from
Ossenkopf & Henning (1994). These "OH5'' opacities are the only ones that yield
dust masses consistent with C17O measurements of AFGL 2591, where
CO depletion is known to be negligible (van der Tak et al. 1999). To constrain  ,
the CS and C34S line spectrum of MonR2 IRS3 was modeled
with the Monte Carlo program. Besides our own JCMT measurements
(Table 5), data from Tafalla et al. (1997) and Choi et al. (2000)
were used. The best fit was obtained for
,
the CS and C34S line spectrum of MonR2 IRS3 was modeled
with the Monte Carlo program. Besides our own JCMT measurements
(Table 5), data from Tafalla et al. (1997) and Choi et al. (2000)
were used. The best fit was obtained for
 (Table 8), with a reduced
(Table 8), with a reduced
 over 11 degrees
of freedom, although
over 11 degrees
of freedom, although
 (
(
 )
and
)
and
 (
(
 )
also give acceptable fits. Radial profiles of 350
)
also give acceptable fits. Radial profiles of 350  m
dust emission for our sources indicate larger values of
m
dust emission for our sources indicate larger values of  ,
by
up to 0.5, than CS line emission (Mueller et al. 2002). If this difference
holds more generally, it may reflect a reduced CS abundance at small
radii, although the models of Sect. 5.2 do not predict
that.
,
by
up to 0.5, than CS line emission (Mueller et al. 2002). If this difference
holds more generally, it may reflect a reduced CS abundance at small
radii, although the models of Sect. 5.2 do not predict
that.
Table 8:
Power law model
 for the density structure of MonR2 IRS3.
for the density structure of MonR2 IRS3.
Table 9 lists the results of the radiative transfer
analysis, with the same source ordering as in Table 7.
Abundances, if assumed constant, are  10-8 for OCS,
10-8 for OCS,
 10-9 for H2S, H2CS, SO and SO2, and
10-9 for H2S, H2CS, SO and SO2, and  10-10 for
HCS+ and NS. These trends are the same as found above for
beam-averaged column densities (Sect. 3.3); the Monte Carlo
analysis does not change results by factors of more than a few.
Except for SO, SO2 and OCS, the source-to-source spread in the
abundances is less than a factor of 10, and not related to
temperature. These molecules (CS, H2S, H2CS, NS and HCS+) appear
to trace the chemically inactive outer envelope (T<100 K), at least
in the observed transitions. However, the high OCS abundances occur
all in warm sources.
10-10 for
HCS+ and NS. These trends are the same as found above for
beam-averaged column densities (Sect. 3.3); the Monte Carlo
analysis does not change results by factors of more than a few.
Except for SO, SO2 and OCS, the source-to-source spread in the
abundances is less than a factor of 10, and not related to
temperature. These molecules (CS, H2S, H2CS, NS and HCS+) appear
to trace the chemically inactive outer envelope (T<100 K), at least
in the observed transitions. However, the high OCS abundances occur
all in warm sources.
Table 9:
Molecular abundances and abundance ratios: outer envelope.
![\begin{figure}
\par\resizebox{13.1cm}{!}{\includegraphics[clip]{ms3386.f4}} \end{figure}](/articles/aa/full/2003/47/aa3386/Timg160.gif) |
Figure 4:
Observations of SO (open circles; envelope component only), and predictions
from Monte Carlo models (filled dots). Line fluxes have been converted
to upper state column densities following Helmich et al. (1994). The
estimated error on the line fluxes is a factor of two, due to
the uncertain contribution from high-velocity gas. |
| Open with DEXTER |
For SO and SO2, the number of detected lines is large enough to
investigate their abundances as a function of radius.
Figure 4 shows that the Monte Carlo models fit the SO
lines uniformly well over the observed range of energy levels for all
sources except W 33 A: there is no indication for changes in the SO
abundance between T=30 and 90 K. In the case of W 33 A, some of
the observed values appear to be higher than the models, which may be
due to an uncertain high-velocity contribution for these
lines. Observations of higher-excitation SO lines ( near
430 GHz or
near
430 GHz or  near 473 GHz) would be valuable to investigate
the effect of evaporating ice mantles on the abundance of SO.
near 473 GHz) would be valuable to investigate
the effect of evaporating ice mantles on the abundance of SO.
![\begin{figure}
\par\resizebox{13.16cm}{!}{\includegraphics[clip]{ms3386.f5}} \end{figure}](/articles/aa/full/2003/47/aa3386/Timg163.gif) |
Figure 5:
As previous figure, for SO2. Triangles indicate models
with a temperature-dependent SO2 abundance. |
| Open with DEXTER |
Figure 5 shows the results of the models for SO2.
Models with SO2/H2
 fit the lines with
fit the lines with
 K within factors of a few. However, these models underproduce the
lines with
K within factors of a few. However, these models underproduce the
lines with
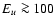 K by factors of
K by factors of  100. This
discrepancy is best seen for W3 IRS5, W33A and AFGL 2591, where the
most SO2 lines are detected. For these sources, we have run models
where the SO2 abundance increases sharply ("jumps'') when T>100 K.
These models are shown as triangles in Fig. 5 and
quantified in Table 10. The SO2 abundance increases by
a factor of 100 in the case of W3 IRS5 and W33A, and by a factor of
1000 for AFGL 2591. These models reproduce all observed SO2 lines
to factors
100. This
discrepancy is best seen for W3 IRS5, W33A and AFGL 2591, where the
most SO2 lines are detected. For these sources, we have run models
where the SO2 abundance increases sharply ("jumps'') when T>100 K.
These models are shown as triangles in Fig. 5 and
quantified in Table 10. The SO2 abundance increases by
a factor of 100 in the case of W3 IRS5 and W33A, and by a factor of
1000 for AFGL 2591. These models reproduce all observed SO2 lines
to factors  10. The residuals are considerably bigger than the
expected error bars of factors of 2...3, mainly due to the uncertain
high-velocity contribution, which suggests that more detailed modeling
is needed. However, these "jump'' models fit the data much better than
models where the SO2 abundance is constant. The SO2 abundances
derived from the high-excitation lines are similar to those based on
the infrared data, indicating that the same gas is probed. The data
for the other sources are consistent with similar abundance jumps, but
not enough high-excitation SO2 lines have been detected to constrain
such models. However, the data of AFGL 2136, NGC 7538 IRS1 and MonR2
IRS3 allow estimates of SO2 abundances in their inner envelopes,
which Table 10 also lists.
Except NGC 6334 IRS1, the jumps in the SO2 abundance occur all in
sources with high T(CH3OH), confirming that the abundance
enhancement is related to high temperatures, probably through
ice evaporation.
10. The residuals are considerably bigger than the
expected error bars of factors of 2...3, mainly due to the uncertain
high-velocity contribution, which suggests that more detailed modeling
is needed. However, these "jump'' models fit the data much better than
models where the SO2 abundance is constant. The SO2 abundances
derived from the high-excitation lines are similar to those based on
the infrared data, indicating that the same gas is probed. The data
for the other sources are consistent with similar abundance jumps, but
not enough high-excitation SO2 lines have been detected to constrain
such models. However, the data of AFGL 2136, NGC 7538 IRS1 and MonR2
IRS3 allow estimates of SO2 abundances in their inner envelopes,
which Table 10 also lists.
Except NGC 6334 IRS1, the jumps in the SO2 abundance occur all in
sources with high T(CH3OH), confirming that the abundance
enhancement is related to high temperatures, probably through
ice evaporation.
Table 10:
Models with SO2 abundance "jumps''.
The models discussed so far assumed a static envelope and aimed to
describe the low-velocity gas. To investigate whether the
high-velocity gas could be due to infalling motions, we have modeled
the SO emission from W3 IRS5 under the assumption of gravitational
infall. In this model, the infall velocity
 varies with
radius R as
varies with
radius R as
 ,
where G is the gravitational constant.
The luminosity of W3 IRS5, if due to a single star, suggests a stellar
mass of
,
where G is the gravitational constant.
The luminosity of W3 IRS5, if due to a single star, suggests a stellar
mass of  30
30  (Vacca et al. 1996), so that
(Vacca et al. 1996), so that
 ranges from 0.45 to 10.66 km s-1 in the model. However, the synthetic
SO line profiles in 14-18'' beams have widths of 5-6 km s-1 FWHM,
much less than the observed value of 13.5 km s-1. Since the other
sources have lower luminosities (hence infall speeds), it seems
unlikely that the high-velocity SO and SO2 emission is solely due to
infall.
ranges from 0.45 to 10.66 km s-1 in the model. However, the synthetic
SO line profiles in 14-18'' beams have widths of 5-6 km s-1 FWHM,
much less than the observed value of 13.5 km s-1. Since the other
sources have lower luminosities (hence infall speeds), it seems
unlikely that the high-velocity SO and SO2 emission is solely due to
infall.
5 Discussion
5.1 Comparison to other objects
Tables 9 and 10 also list molecular
abundances in other regions. Only the case of the low-mass protostar
IRAS 16293 has been analyzed to the same level of detail as done here.
To reduce uncertainties introduced by methodological differences, the
table contains abundances scaled to the value of the commonly observed
CS molecule. Using ratios also facilitates comparison with chemical
models, because these are independent of the total amount of sulphur
in the gas phase, which is not well-understood. Ultraviolet
observations of diffuse clouds toward  Oph indicate that
sulphur is undepleted from its solar abundance of
Oph indicate that
sulphur is undepleted from its solar abundance of
 (Savage & Sembach 1996). However, for dense clouds, it is unclear
what fraction of sulphur is locked up in dust grains.
(Savage & Sembach 1996). However, for dense clouds, it is unclear
what fraction of sulphur is locked up in dust grains.
The ratios observed in our sources differ considerably from those in
PDRs, dark clouds and shocks, indicating that in those regions, other
chemical processes dominate. The ratios in IRAS 16293 are at the low
end of the values found here, suggesting that in low-mass objects,
depletion of molecules onto grains is more important.
In the case of hot cores, both abundance ratios and absolute
abundances are similar to those observed here. The chemical
similarity of our sources with hot cores prompts us to compare our
results with chemical models invoking ice evaporation. Our
assumption is that source-to-source differences in abundances are due
to age differences, rather than to differences in initial physical or
chemical conditions.
5.2 Comparison with chemical models
The sulphur chemistry of hot cores, where ice mantles are evaporating
off dust grains and start a high-temperature chemistry, was studied by
Charnley (1997). The model assumes that the dominant form of sulphur in
the ices is H2S, which after evaporation is destroyed by H and
H3O+ to form S and H3S+. Reactions with OH and O2 make first SO
and then SO2. Some H2S reacts with C+ into CS, which reacts with
OH to make OCS. The sulphur chemistry strongly depends on temperature
through its connection with the oxygen chemistry. At  K,
the SO2 abundance remains factors of 10-100 below that of SO
because O and OH are driven into H2O. Results also depend on the
presence of O2 in the ice mantles. Observational limits on solid
and gaseous O2 (Goldsmith et al. 2000; Pagani et al. 2003; Vandenbussche et al. 1999) do not firmly rule out
either assumption, but do make the model without O2 ice more
plausible. However, this model cannot directly be compared with our
observations, which probe a mixture of warm and cold gas.
K,
the SO2 abundance remains factors of 10-100 below that of SO
because O and OH are driven into H2O. Results also depend on the
presence of O2 in the ice mantles. Observational limits on solid
and gaseous O2 (Goldsmith et al. 2000; Pagani et al. 2003; Vandenbussche et al. 1999) do not firmly rule out
either assumption, but do make the model without O2 ice more
plausible. However, this model cannot directly be compared with our
observations, which probe a mixture of warm and cold gas.
The chemistry of protostellar envelopes was modeled by Doty et al. (2002).
In the inner warm region, a hot core chemistry similar to that of
Charnley (1997) is used, whereas in the cold outer parts, a
low-temperature chemistry including freeze-out is adopted. The
initial conditions of the model depend on temperature to mimic the
effect of ice evaporation. Although Doty et al. adopt the specific
temperature and density structure of AFGL 2591 as modeled by
van der Tak et al. (2000b), their results do not change by factors of more than a
few for our other sources, which have temperature and density
distributions similar to those of AFGL 2591.
The Doty et al. (2002) model has most gas-phase sulphur initially in S at
T<100 K (S/H2
 )
and in H2S at T>100 K
(H2S/H2
)
and in H2S at T>100 K
(H2S/H2
 )
to mimic the effect of ice
evaporation. This choice of initial conditions is consistent with the
nondetection of the [S I] line at 25.249
)
to mimic the effect of ice
evaporation. This choice of initial conditions is consistent with the
nondetection of the [S I] line at 25.249  m toward our sources
with ISO. The upper limit of
m toward our sources
with ISO. The upper limit of  in absorption implies N(S)
in absorption implies N(S)
 cm-2, so that for N(H2)
cm-2, so that for N(H2)
 cm-2,
S/H2
cm-2,
S/H2
 ,
indicating that not all gas-phase sulphur in
the inner envelope can be in atomic form. The inferred abundances
imply that SO2 is one of the dominant sulphur-bearing gas-phase
molecules in the warm inner envelope. However, the sum of all
sulphur-bearing molecules in the gas phase is still much lower than
the value derived for diffuse clouds (Savage & Sembach 1996). Unless
a major sulphur-bearing gas-phase species has been missed in this
survey,
,
indicating that not all gas-phase sulphur in
the inner envelope can be in atomic form. The inferred abundances
imply that SO2 is one of the dominant sulphur-bearing gas-phase
molecules in the warm inner envelope. However, the sum of all
sulphur-bearing molecules in the gas phase is still much lower than
the value derived for diffuse clouds (Savage & Sembach 1996). Unless
a major sulphur-bearing gas-phase species has been missed in this
survey,  90% of the sulphur must be in a solid form which has
not yet been detected.
90% of the sulphur must be in a solid form which has
not yet been detected.
Infrared observations of our sources with ISO-SWS do not support the
assumption that H2S is the main sulphur reservoir of the grain
mantles. Toward W33A, a source with large ice abundances, the
3.98  m band of solid H2S is not detected to
m band of solid H2S is not detected to
 ,
which
using a band strength of
,
which
using a band strength of
 cm mol-1 gives
cm mol-1 gives
 (H2S)
(H2S)
 cm-2, or
cm-2, or  (H2S)/
(H2S)/ (H2O)
<0.03 (W. Schutte, priv. comm.). However, solid OCS was detected
with an abundance of
(H2O)
<0.03 (W. Schutte, priv. comm.). However, solid OCS was detected
with an abundance of  (OCS)/
(OCS)/ (H2O)
(H2O)
 or OCS/H2
or OCS/H2
 (Palumbo et al. 1997). Evaporation of
solid OCS may explain our measured gas-phase OCS abundances which are
otherwise unaccounted for. W 33 A is the only source in our sample
for which both gas-phase and solid OCS have been detected. The
results imply a gas/solid ratio of
(Palumbo et al. 1997). Evaporation of
solid OCS may explain our measured gas-phase OCS abundances which are
otherwise unaccounted for. W 33 A is the only source in our sample
for which both gas-phase and solid OCS have been detected. The
results imply a gas/solid ratio of  0.5, much higher than that
found for e.g. H2O and CO2 in this source
(Boonman & van Dishoeck 2003; Boonman et al. 2003). Observations of both gas-phase and
solid-state OCS toward other sources are needed to determine the role
of grain-mantle evaporation. Observations of OCS lines from a wide
range of energy levels may reveal an abundance increase at
0.5, much higher than that
found for e.g. H2O and CO2 in this source
(Boonman & van Dishoeck 2003; Boonman et al. 2003). Observations of both gas-phase and
solid-state OCS toward other sources are needed to determine the role
of grain-mantle evaporation. Observations of OCS lines from a wide
range of energy levels may reveal an abundance increase at  K, as indeed found for IRAS 16293 (Schöier et al. 2002). The low
K, as indeed found for IRAS 16293 (Schöier et al. 2002). The low
 of H2S and the high value of OCS (Sect. 3.2) support these
conclusions.
of H2S and the high value of OCS (Sect. 3.2) support these
conclusions.
The effect of evaporating OCS-rich ice mantles on hot core chemistry
is explored by Doty et al. (2003). Briefly, the gas-phase abundances of
CS, HCS+ and OCS increase about linearly with the fraction of solid
OCS, while those of H2S, SO and SO2 decrease about linearly. The
H2CS molecule is not affected much. Since the gas-phase chemistry of
CS, HCS+ and OCS depends only weakly on time, the use of sulphur
molecules as chemical clock diminishes when H2S is only a minor
sulphur carrier in the ice mantles. However, the "jump'' models for
SO2 (Table 10) indicate that either H2S or SO2 is
present in the grain mantles. The limits on solid SO2 from infrared
observations are not very stringent (Boogert et al. 1998).
We have compared our observations to the models of Doty et al. (2002),
modified to have OCS in the evaporating ice mantles (S. Doty, priv.
comm.). This model reproduces our observed abundances of CS, SO and
H2CS in the outer envelopes (Table 9) to within factors
of a few for chemical ages of  105 yr. The abundances of SO2
in the inner envelopes (Table 10) are also reproduced for
the same chemical age. The model abundances of HCS+ and SO2 in the
outer envelopes are factors of
105 yr. The abundances of SO2
in the inner envelopes (Table 10) are also reproduced for
the same chemical age. The model abundances of HCS+ and SO2 in the
outer envelopes are factors of  10 below the observed values,
which may be due to the adopted initial conditions, or the chemical
network, which is based on the UMIST reaction rate database
(Le Teuff et al. 2000)
10 below the observed values,
which may be due to the adopted initial conditions, or the chemical
network, which is based on the UMIST reaction rate database
(Le Teuff et al. 2000)![[*]](/icons/foot_motif.gif) . The outer envelope
abundances of H2S and OCS are underproduced even more (factors of
. The outer envelope
abundances of H2S and OCS are underproduced even more (factors of
 100). However, the small number of observed lines forced us to
assume constant abundances for these species, while they may in fact
be confined to the inner envelopes.
100). However, the small number of observed lines forced us to
assume constant abundances for these species, while they may in fact
be confined to the inner envelopes.
5.3 The role of shock chemistry
As an alternative to ice evaporation models, we consider the effect of
non-dissociative shocks on the molecular envelopes surrounding the
protostars. Such shocks occur by the interaction of the molecular
outflows of protostars with their envelopes. Shocks fast enough to
dissociate H2 ( km s-1) would have a major impact on their
molecular composition which our data do not indicate. Although our
sources show outflows faster than that, both in infrared absorption
(Mitchell et al. 1991) and in submillimeter emission
(Mitchell et al. 1992; Mitchell & Hasegawa 1991) of CO, their filling factor must be
small.
km s-1) would have a major impact on their
molecular composition which our data do not indicate. Although our
sources show outflows faster than that, both in infrared absorption
(Mitchell et al. 1991) and in submillimeter emission
(Mitchell et al. 1992; Mitchell & Hasegawa 1991) of CO, their filling factor must be
small.
Our observed SO, SO2 and H2S abundances in the outer envelopes are
consistent with those in postshock gas if sulphur was initially in
molecular form (Leen & Graff 1988). The observed similarity of these
abundances is not reproduced by the Pineau des Forêts et al. (1993) model which has
most sulphur initially in atomic form. However, the two models are
hard to compare because one is a two-fluid treatment, while the other
treats neutral and charged particles as one fluid. Before concluding
that postshock gas reproduces our data, we recommend that a two-fluid
calculation is performed with most sulphur initially in molecular
form.
Two other molecules suggest that the envelopes of young high-mass
stars may have been processed by shocks, although neither one
conclusively. First, ISO-SWS data show low ratios of gas-phase to
solid-state CO2 ( 0.1; van Dishoeck 1998) and the CO2 gas
phase abundance remains low through the 100-300 K temperature regime.
After evaporating off grains, CO2 must be promptly destroyed, which
shocks can do in reactions with H (Charnley & Kaufman 2000) or perhaps H2
(Doty et al. 2002; Talbi & Herbst 2002). Second, Hatchell & Viti (2002) measured NS/CS ratios of
0.02-0.05 in a sample of six hot cores and interpreted these as
evidence for shocks. The main reactions to form NS require SH and NH
which are produced from OH. Shocks use OH to form H2O and suppress
the production of NS. The values of NS/CS = 0.001-0.01 measured here
may indicate that shocks play a role too. However, for
0.1; van Dishoeck 1998) and the CO2 gas
phase abundance remains low through the 100-300 K temperature regime.
After evaporating off grains, CO2 must be promptly destroyed, which
shocks can do in reactions with H (Charnley & Kaufman 2000) or perhaps H2
(Doty et al. 2002; Talbi & Herbst 2002). Second, Hatchell & Viti (2002) measured NS/CS ratios of
0.02-0.05 in a sample of six hot cores and interpreted these as
evidence for shocks. The main reactions to form NS require SH and NH
which are produced from OH. Shocks use OH to form H2O and suppress
the production of NS. The values of NS/CS = 0.001-0.01 measured here
may indicate that shocks play a role too. However, for
 yr, the Doty et al. (2002) model also predicts NS/CS
yr, the Doty et al. (2002) model also predicts NS/CS
 ,
so this ratio cannot be used to demonstrate the influence of shocks.
,
so this ratio cannot be used to demonstrate the influence of shocks.
6 Conclusions
Submillimeter observations of SO, SO2, H2S, H2CS, OCS, NS and
HCS+ toward nine embedded massive stars show that:
- 1.
- High-velocity gas contributes 10-50% to the CS, SO and SO2
emission in 15-20'' beams. This gas may either trace the outflows
which are also seen in CO, or they may trace infall motions.
- 2.
- The SO2 abundance increases from dark cloud levels in the
outer envelope (T<100 K) to levels seen in hot cores and shocks in
the inner envelope (T>100 K).
- 3.
- Molecular abundances are consistent with a model of ice
evaporation in an envelope with gradients in temperature and density
for a chemical age of
 105 yr.
105 yr.
- 4.
- The high observed abundance of OCS, the fact that
 (OCS)
(OCS)
 (H2S), and the detection of solid OCS and limit on
solid H2S all suggest that OCS is a major sulphur carrier in grain
mantles, rather than H2S.
(H2S), and the detection of solid OCS and limit on
solid H2S all suggest that OCS is a major sulphur carrier in grain
mantles, rather than H2S.
- 5.
- For most other sulphur-bearing molecules, the source-to-source
abundance variations by factors of up to 10 do not correlate with
previously established evolutionary trends in temperature tracers.
These species probe the chemically inactive outer envelope. Our
data set does not constrain the abundances of H2S and SO in the
inner envelope, which, together with SO2, are required to use
sulphur as a clock.
These conclusions could be followed up by, respectively:
- 1.
- interferometric observations at
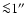 resolution of SO
lines from a wide range of energy levels;
resolution of SO
lines from a wide range of energy levels;
- 2.
- similar interferometric observations of SO2;
- 3.
- updating the model of Leen & Graff (1988) using a two-fluid
treatment, and extending it to later times;
- 4.
- a multi-line study of OCS, covering energy levels well above and
well below 100 K;
- 5.
- observations of high-excitation SO and H2S lines, e.g. SO 430 and 473 GHz and H2S 369 GHz.
Items 3 and 4 are within the capabilities of current computational and
observational facilities. The CSO and JCMT may be able to carry out
item 5 in very good weather, but the future Atacama Pathfinder
Experiment (APEX) can benefit from an even better site. Current
interferometers are not sensitive enough for items 1 and 2, which
require observing several lines and several sources. However, future
instruments such as the Combined Array for Research into Millimeter
Astronomy (CARMA) and the Atacama Large Millimeter Array (ALMA) will
be well-suited for these projects.
Acknowledgements
The authors thank the JCMT and IRAM staffs for their support,
Malcolm Walmsley, Holger Müller, Jennifer Hatchell and Xander
Tielens for useful discussions, and Willem Schutte, Fred Lahuis and
Steve Doty for input. Astrochemistry in Leiden is supported by the
Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO)
through grant 614-41-003 and a Spinoza award.
-
Ball, C. D., Mengel, M., De Lucia, F. C., & Woon, D. E. 1999, J. Chem. Phys., 111, 8893
In the text
-
Beers, Y., Klein, X., Kirchhoff, W. H., & Johnson, D. R. 1972, J. Mol. Sp.,
44, 553
In the text
-
Blake, G. A., Sutton, E. C., Masson, C. R., & Phillips, T. G. 1987,
ApJ, 315, 621
In the text
NASA ADS
-
Boogert, A. C. A., Ehrenfreund, P., Gerakines, P. A., et al. 2000,
A&A, 353, 349
In the text
NASA ADS
-
Boogert, A. C. A., Helmich, F. P., van Dishoeck, E. F., et al. 1998,
A&A, 336, 352
In the text
NASA ADS
-
Boonman, A. M. S., & van Dishoeck, E. F. 2003, A&A, 403, 1003
In the text
NASA ADS
-
Boonman, A. M. S., van Dishoeck, E. F., Lahuis, F., & Doty, S. D. 2003,
A&A, 399, 1063
In the text
NASA ADS
-
Boonman, A. M. S., Wright, C. M., & van Dishoeck, E. F. 1999, in 3rd
Cologne-Zermatt Symp., ed. V. Ossenkopf, J. Stutzki, & G.
Winnewisser, 275
-
Buckle, J. V., & Fuller, G. A. 2003, A&A, 399, 567
In the text
NASA ADS
-
Charnley, S. B. 1997, ApJ, 481, 396
In the text
NASA ADS
-
Charnley, S. B., & Kaufman, M. J. 2000, ApJ, 529, L111
In the text
NASA ADS
-
Choi, M. 2002, ApJ, 575, 900
In the text
NASA ADS
-
Choi, M., Evans, N. J., Tafalla, M., & Bachiller, R. 2000, ApJ, 538,
738
In the text
NASA ADS
-
Doty, S. D., Schöier, F. L., & van Dishoeck, E. F. 2003, A&A,
submitted
In the text
-
Doty, S. D., van Dishoeck, E. F., van der Tak, F. F. S., & Boonman,
A. M. S. 2002, A&A, 389, 446
In the text
NASA ADS
-
Giannakopoulou, J., Mitchell, G. F., Hasegawa, T. I., Matthews, H. E.,
& Maillard, J. 1997, ApJ, 487, 346
In the text
NASA ADS
-
Goldsmith, P. F., Melnick, G. J., Bergin, E. A., et al. 2000, ApJ,
539, L123
In the text
NASA ADS
-
Green, S. 1995, ApJS, 100, 213
In the text
NASA ADS
-
Green, S., Maluendes, S., & McLean, A. D. 1993, ApJS, 85, 181
In the text
NASA ADS
-
Hatchell, J., Thompson, M. A., Millar, T. J., & MacDonald, G. H.
1998a, A&AS, 133, 29
In the text
NASA ADS
-
Hatchell, J., Thompson, M. A., Millar, T. J., & MacDonald, G. H.
1998b, A&A, 338, 713
In the text
NASA ADS
-
Hatchell, J., & Viti, S. 2002, A&A, 381, L33
In the text
NASA ADS
-
Helmich, F. P., Jansen, D. J., de Graauw, T., Groesbeck, T. D., & van Dishoeck, E. F. 1994, A&A, 283, 626
In the text
NASA ADS
-
Helmich, F. P., & van Dishoeck, E. F. 1997, A&AS, 124, 205
In the text
NASA ADS
-
Henning, T., Chini, R., & Pfau, W. 1992, A&A, 263, 285
In the text
NASA ADS
-
Hogerheijde, M. R., & van der Tak, F. F. S. 2000, A&A, 362, 697
In the text
NASA ADS
-
Jansen, D. J., Spaans, M., Hogerheijde, M. R., & van Dishoeck, E. F.
1995, A&A, 303, 541
NASA ADS
-
Keane, J. V., Boonman, A. M. S., Tielens, A. G. G. M., & van Dishoeck,
E. F. 2001, A&A, 376, L5
In the text
NASA ADS
-
Langer, W. D., van Dishoeck, E. F., Bergin, E. A., et al. 2000,
Protostars and Planets IV, 29
In the text
-
Le Teuff, Y. H., Millar, T. J., & Markwick, A. J. 2000, A&AS, 146, 157
In the text
NASA ADS
-
Lee, C., Mundy, L. G., Stone, J. M., & Ostriker, E. C. 2002, ApJ,
576, 294
In the text
NASA ADS
-
Leen, T. M., & Graff, M. M. 1988, ApJ, 325, 411
In the text
NASA ADS
-
McCutcheon, W. H., Sandell, G., Matthews, H. E., et al. 2000, MNRAS,
316, 152
In the text
NASA ADS
-
Minh, Y. C., Irvine, W. M., McGonagle, D., & Ziurys, L. M. 1990, ApJ,
360, 136
NASA ADS
-
Mitchell, G. F. 1984, ApJ, 287, 665
In the text
NASA ADS
-
Mitchell, G. F., & Hasegawa, T. I. 1991, ApJ, 371, L33
In the text
NASA ADS
-
Mitchell, G. F., Hasegawa, T. I., & Schella, J. 1992, ApJ, 386, 604
In the text
NASA ADS
-
Mitchell, G. F., Maillard, J.-P., & Hasegawa, T. I. 1991, ApJ, 371, 342
In the text
NASA ADS
-
Mueller, K. E., Shirley, Y. L., Evans, N. J., & Jacobson, H. R. 2002,
ApJS, 143, 469
In the text
NASA ADS
-
Nummelin, A., Bergman, P., Hjalmarson, Å., et al. 2000, ApJS, 128,
213
In the text
NASA ADS
-
Ohishi, M., Irvine, W. M., & Kaifu, N. 1992, in IAU Symp., 150,
Astrochemistry of Cosmic Phenomena, 171
-
Ossenkopf, V., & Henning, T. 1994, A&A, 291, 943
In the text
NASA ADS
-
Pagani, L., Olofsson, A. O. H., Bergman, P., et al. 2003, A&A, 402,
L77
In the text
NASA ADS
-
Palumbo, M. E., Geballe, T. R., & Tielens, A. G. G. M. 1997, ApJ, 479,
839
In the text
NASA ADS
-
Pickett, H. M., Poynter, R. L., Cohen, E. A., et al. 1998, J. Quant. Spectrosc. Radiat. Transfer, 60, 883
In the text
-
Pineau des Forêts, G., Roueff, E., Schilke, P., & Flower, D. R.
1993, MNRAS, 262, 915
In the text
NASA ADS
-
Savage, B. D., & Sembach, K. R. 1996, ARA&A, 34, 279
In the text
NASA ADS
-
Schilke, P., Groesbeck, T. D., Blake, G. A., & Phillips, T. G. 1997,
ApJS, 108, 301
In the text
NASA ADS
-
Schöier, F. L., Jørgensen, J. K., van Dishoeck, E. F., & Blake,
G. A. 2002, A&A, 390, 1001
In the text
NASA ADS
-
Sutton, E. C., Peng, R., Danchi, W. C., et al. 1995, ApJS, 97, 455
In the text
NASA ADS
-
Tafalla, M., Bachiller, R., Wright, M. C. H., & Welch, W. J. 1997,
ApJ, 474, 329
In the text
NASA ADS
-
Talbi, D., & Herbst, E. 2002, A&A, 386, 1139
In the text
NASA ADS
-
Turner, B. E. 1996, ApJ, 468, 694
In the text
NASA ADS
-
Vacca, W. D., Garmany, C. D., & Shull, J. M. 1996, ApJ, 460, 914
In the text
NASA ADS
-
van der Tak, F. F. S., van Dishoeck, E. F., & Caselli, P.
2000a, A&A, 361, 327
In the text
NASA ADS
-
van der Tak, F. F. S., van Dishoeck, E. F., Evans, N. J., Bakker,
E. J., & Blake, G. A. 1999, ApJ, 522, 991
In the text
NASA ADS
-
van der Tak, F. F. S., van Dishoeck, E. F., Evans, N. J., & Blake,
G. A. 2000b, ApJ, 537, 283
In the text
NASA ADS
-
van Dishoeck, E. F. 1998, Faraday Discussion, 109, 31
In the text
-
van Dishoeck, E. F. 2001, The Promise of the Herschel Space
Observatory, ed. G. L. Pilbratt, J. Cernicharo, A. M. Heras, T. Prusti, &
R. Harris, ESA-SP, 460, 185
In the text
-
Vandenbussche, B., Ehrenfreund, P., Boogert, A. C. A., et al. 1999,
A&A, 346, L57
In the text
NASA ADS
Copyright ESO 2003
![\begin{figure}
\par\resizebox{16.2cm}{!}{\includegraphics[angle=-90,clip]{ms3386.f1}} \end{figure}](/articles/aa/full/2003/47/aa3386/Timg11.gif)
![\begin{figure}
\par\resizebox{8.8cm}{!}{\includegraphics[angle=-90,clip]{ms3386.f2}} \end{figure}](/articles/aa/full/2003/47/aa3386/Timg81.gif)
![\begin{figure}
\par\resizebox{8.5cm}{!}{\includegraphics[clip]{ms3386.f3}} \end{figure}](/articles/aa/full/2003/47/aa3386/Timg139.gif)
![\begin{figure}
\par\resizebox{13.1cm}{!}{\includegraphics[clip]{ms3386.f4}} \end{figure}](/articles/aa/full/2003/47/aa3386/Timg160.gif)
![\begin{figure}
\par\resizebox{13.16cm}{!}{\includegraphics[clip]{ms3386.f5}} \end{figure}](/articles/aa/full/2003/47/aa3386/Timg163.gif)