A&A 408, 193-204 (2003)
DOI: 10.1051/0004-6361:20030916
C. Jäger1 - J. Dorschner1 - H. Mutschke1 - Th. Posch2 - Th. Henning3
1 - Astrophysikalisches Institut und
Universitäts-Sternwarte (AIU), Schillergäßchen 2-3,
07745 Jena, Germany
2 - Institut für Astronomie,
Türkenschanzstraße 17, 1180 Wien, Austria
3 - Max-Planck-Institut für Astronomie,
Königstuhl 17, 69117 Heidelberg, Germany
Received 31 May 2002 / Accepted 4 June 2003
Abstract
Amorphous silicate particles are generally assumed to be the
main dust component in the envelopes of oxygen-rich evolved stars
and may be considered the precursors of the pure crystalline
enstatite and forsterite particles detected by ISO.
We present optical constants in the broad wavelength
range 0.2-500 ![]() m for a unique series of pure amorphous Mg-silicates
(Mg/Si in the range 0.7-2.4). They have been prepared by the sol-gel process, a
chemical technique based on the condensation of Mg- and
Si-hydroxides in a liquid phase. The salient feature of these Mg-silicates
is the very small content of Si-OH bonds in the
silicate network, which considerably reduces the activation energy
of crystallization and, thus, decreases the temperature threshold
for crystallization as well as crystallization time. The
astrophysical relevance of our sol-gel silicates is shown by
a comparison of optically thin model spectra based on
dust emissivities with ISO-SWS spectra of AGB stars
and with 10
m for a unique series of pure amorphous Mg-silicates
(Mg/Si in the range 0.7-2.4). They have been prepared by the sol-gel process, a
chemical technique based on the condensation of Mg- and
Si-hydroxides in a liquid phase. The salient feature of these Mg-silicates
is the very small content of Si-OH bonds in the
silicate network, which considerably reduces the activation energy
of crystallization and, thus, decreases the temperature threshold
for crystallization as well as crystallization time. The
astrophysical relevance of our sol-gel silicates is shown by
a comparison of optically thin model spectra based on
dust emissivities with ISO-SWS spectra of AGB stars
and with 10 ![]() m emission profiles of such stars obtained
by ground-based spectroscopy. As paradigmatic
cases of AGB spectra with respect to the appearance of the
silicate bands, TY Dra (slender bands and deep trough between them)
and R Cas (broad bands and widely filled-up trough) were used, for
which ISO-SWS spectra are available. The dust
emissivity derived from TY Dra can be excellently reproduced by the
models, suggesting that the dust grains consist indeed of pure
amorphous Mg-silicates. Satisfactory agreement was also found with
the mean 10
m emission profiles of such stars obtained
by ground-based spectroscopy. As paradigmatic
cases of AGB spectra with respect to the appearance of the
silicate bands, TY Dra (slender bands and deep trough between them)
and R Cas (broad bands and widely filled-up trough) were used, for
which ISO-SWS spectra are available. The dust
emissivity derived from TY Dra can be excellently reproduced by the
models, suggesting that the dust grains consist indeed of pure
amorphous Mg-silicates. Satisfactory agreement was also found with
the mean 10 ![]() m profiles of some groups of AGB stars and supergiants.
Spectra with strong dust emission in the silicate trough like R Cas require
additional contributions by other dust components, probably oxides. A
rough orientation on the spectral properties of such potential
trough opacity contributors has been obtained by subtracting a
pure silicate spectrum (TY Dra) from a spectrum with a nearly
filled trough and a less pronounced 20
m profiles of some groups of AGB stars and supergiants.
Spectra with strong dust emission in the silicate trough like R Cas require
additional contributions by other dust components, probably oxides. A
rough orientation on the spectral properties of such potential
trough opacity contributors has been obtained by subtracting a
pure silicate spectrum (TY Dra) from a spectrum with a nearly
filled trough and a less pronounced 20 ![]() m band (R Cas). In
agreement with other amorphous silicates, the spectral index of
the new silicate analogues amounts to -2.
m band (R Cas). In
agreement with other amorphous silicates, the spectral index of
the new silicate analogues amounts to -2.
Key words: infrared: stars - methods: laboratory - infrared: ISM - stars: circumstellar matter - line: identification
Spectra obtained by the Infrared Space Observatory (ISO) have surprisingly pointed to clear evidence of crystalline silicates. Observational evidence for the presence of crystalline silicates was found in the spectra of circumstellar dust around Herbig Ae/Be stars (Waelkens et al. 1996), low-mass evolved stars with high mass-loss including planetary nebulae (Justtanont et al. 1996; Waters et al. 1996) and Luminous Blue Variables (LBVs) (Waters et al. 1997). The circumstellar spectra of crystalline silicates exhibited great similarity with the spectrum of comet Hale-Bopp (Crovisier et al. 1997). From the comparison between laboratory spectra of pyroxene- and olivine-type silicates and astronomical spectra, it has been concluded that only completely iron-free magnesium silicates like forsterite and enstatite can account for the observed band positions (Bowey et al. 2002; Molster et al. 2002b; Jäger et al. 1998).
Recently, forsterite has been found among the first presolar silicates that were identified in interplanetary dust particles (Messenger et al. 2002) due to a large 17O excess and moderate 18O depletion (which is characteristic of H burning). This forsterite grain has been proposed to originate from an AGB star. Other silicate grains enriched in 17O and 18O point to an origin in high metallicity stars and type II supernovae. Due to their small sizes, chemically and isotopically similar grains could not be fully characterized, but it has been shown that they are rich in O, Si and Mg.
The main dust component in different astronomical environments, though, remains amorphous magnesium-iron silicates (Demyk et al. 1999; Bouwman et al. 2001; Molster et al. 2002a,b,1999). The crystalline magnesium silicates have probably been formed by thermal annealing. The conversion of amorphous magnesium silicates to crystalline components can be explained by means of annealing processes occurring close to the condensation zones (Fabian et al. 2000; Hallenbeck et al. 1998). However, the annealing temperature necessary to convert the amorphous into the crystalline silicates at a timescale of hours to days is 1000 K. Unless the crystalline Mg-silicates can condense directly from the gas phase, nearly iron-free amorphous silicates should be the precursors of crystalline forsterite and enstatite. Additionally, Rietmeijer et al. (1999) demonstrated the formation of either pure magnesium or iron silicates in vapor-phase condensation experiments. In particular, no mixed Mg-Fe-silicate grains have been produced in the condensation experiment.
Therefore, we concentrated our experiments on amorphous, iron-free magnesium silicates. This does not mean that we consider Mg-silicates as the only silicate dust component. Mixed Mg-Fe-silicates can be formed after the condensation of Mg-silicates by solid-state reactions with iron or iron-containing species.
With some exceptions (Hallenbeck et al. 2000; Dorschner et al. 1995; Scott & Duley 1996), optical
constants of amorphous Mg-silicates are rather rare in the
literature. It is the aim of this paper to provide optical data of
amorphous silicates with varying MgO/SiO2 ratios which cover the
whole wavelength range important for the interpretation of ISO spectra.
This investigation includes Mg0.7SiO2.7, MgSiO3, Mg1.5SiO3.5,
Mg2SiO4, and Mg2.4SiO4.4. Two of the produced nonstoichiometric materials
correspond to the metastable eutectic Mg-silicates compositions Mg3Si2O7and Mg6Si8O22 reported to condense preferably in the
magnesium silicate binary system by Rietmeijer et al. (1999). Furthermore, the influence of the
Mg/Si ratio on band positions and strength ratios of the 10 and 20 ![]() m bands has been studied. The spectroscopy is
supplemented by a careful analytical characterization of the
samples.
m bands has been studied. The spectroscopy is
supplemented by a careful analytical characterization of the
samples.
The sol-gel technique used for the synthesis of magnesium silicate samples is not only interesting for the production of high-melting silicates. It also provides materials bearing chemical properties expected for cosmic silicates. In fact, the formation of magnesium silicates with a small content of isolated Si-OH or Mg-OH bonds can be considered as highly probable, since H2O, the most abundant oxygen-bearing molecule, participates in the formation of olivine- and pyroxene-type silicates (Gail & Sedlmayr 1998). These silicate materials show dissenting material properties in comparison to the commonly produced silicates, particularly with regard to their crystallization behaviour. Therefore we completed our experiments with investigations of the annealing behaviour of the synthesized magnesium silicate samples.
The sol-gel process is based on the chemical polymerization of silicates in a liquid phase at low temperatures. Metal organic compounds like tetraethoxysiloxane (TEOS) and magnesium methylate Mg(OCH3)2 served as precursors. The metal organic materials have to be soluble in water and alcohol. The polymerization runs in two steps. The first step is the hydrolysis of the precursors and the formation of hydroxides, followed by the condensation of the hydroxides (Si(OH)4 and Mg(OH)2). It leads to the formation of a three-dimensional magnesium silicate network. Hydrolysis and condensation are chemical equilibrium reactions which run in competition. In order to avoid phase separations and the formation of inhomogeneous silicate material, it is important to choose silicon and magnesium components with nearly the same hydrolysis and condensation rates.
Compositions up to 0.7 mol MgO in the system have been synthesized
with magnesium chloride or magnesium nitrate and
tetraethoxysiloxane. Metal oxide (MeO) contents larger than 50 Mol% MgO (![]() )
cannot be produced from anorganic magnesium compounds since
they lead to the precipitation of the salt during the reaction
(Burlitch et al. 1991).
)
cannot be produced from anorganic magnesium compounds since
they lead to the precipitation of the salt during the reaction
(Burlitch et al. 1991).
In order to produce amorphous magnesium silicates
MgxSiO2+x (for
![]() ), we used an advancement of
the H2O2-assisted sol-gel reaction of magnesium methylate
(Mg(OCH3)2) and TEOS based on the synthesis described by
Burlitch et al. (1991) (see also Agladze et al. 1996). The hydrogen
peroxide serves as a proton provider and a catalyst. The synthesis
has to be performed under a purified Ar-atmosphere to avoid the
precipitation of insoluble Mg(OH)(OCH3) and phase separation of MgO.
The removal of the solvents methanol and water from
the produced gels has been run in a rotary evaporation at reduced
pressure. The remaining magnesium silicate powder was heated in
order to achieve a densification of the silicate framework and to
remove the porosity of the grains. The amorphousness of the
samples has been proven by means of X-ray diffraction. The
stoichiometry, purity, and phase homogeneity as well as the
densification process could be determined by using the
Transmission Electron Microscope (TEM) and the Scanning Electron
Microscope (SEM) combined with the Electron Dispersive X-ray (EDX)
analysis. The samples' compositions were exactly
determined by EDX measurements. The compositional results from EDX
were carefully compared with corresponding well-analyzed standard
samples comprising crystalline and amorphous materials with
compositions from Mg0.5SiO2.5...Mg2SiO4. In case
of two samples, wet-chemical analyses were also performed in order to
confirm our EDX results. The homogeneity and purity of the samples
were additionally checked by TEM analysis combined with EDX. For an overview
of the compositions, see Table 1.
), we used an advancement of
the H2O2-assisted sol-gel reaction of magnesium methylate
(Mg(OCH3)2) and TEOS based on the synthesis described by
Burlitch et al. (1991) (see also Agladze et al. 1996). The hydrogen
peroxide serves as a proton provider and a catalyst. The synthesis
has to be performed under a purified Ar-atmosphere to avoid the
precipitation of insoluble Mg(OH)(OCH3) and phase separation of MgO.
The removal of the solvents methanol and water from
the produced gels has been run in a rotary evaporation at reduced
pressure. The remaining magnesium silicate powder was heated in
order to achieve a densification of the silicate framework and to
remove the porosity of the grains. The amorphousness of the
samples has been proven by means of X-ray diffraction. The
stoichiometry, purity, and phase homogeneity as well as the
densification process could be determined by using the
Transmission Electron Microscope (TEM) and the Scanning Electron
Microscope (SEM) combined with the Electron Dispersive X-ray (EDX)
analysis. The samples' compositions were exactly
determined by EDX measurements. The compositional results from EDX
were carefully compared with corresponding well-analyzed standard
samples comprising crystalline and amorphous materials with
compositions from Mg0.5SiO2.5...Mg2SiO4. In case
of two samples, wet-chemical analyses were also performed in order to
confirm our EDX results. The homogeneity and purity of the samples
were additionally checked by TEM analysis combined with EDX. For an overview
of the compositions, see Table 1.
Table 1: Composition of the magnesium silicate samples used for reflection measurements derived by EDX measurements in the SEM and TEM.
The final powder products have been pressed into very dense
pellets at pressures of about 200 t/cm2 load. The pellets were
embedded in an epoxide resin and their surfaces were ground and
polished. Specular reflectance measurements, at nearly normal
incidence, have been performed at the embedded and polished
samples from the UV up to the FIR range (0.2-200 ![]() m). In the
high frequency range a Perkin Elmer Lambda 19 UV/VIS/NIR
spectrometer, covering the region between 0.2 and 2.5
m). In the
high frequency range a Perkin Elmer Lambda 19 UV/VIS/NIR
spectrometer, covering the region between 0.2 and 2.5 ![]() m, was
applied. The spectral resolution depends on the wavelength and was
0.5 nm in the UV/VIS range. An aluminium mirror of calibrated reflectance was used
as reference. The IR reflectance measurements in the range between 2 and 300
m, was
applied. The spectral resolution depends on the wavelength and was
0.5 nm in the UV/VIS range. An aluminium mirror of calibrated reflectance was used
as reference. The IR reflectance measurements in the range between 2 and 300 ![]() m were performed by means of a Bruker FTIR
spectrometer 113v. In this spectral region a gold mirror reference
came into operation. The spectral resolution in the IR was 2 cm-1 which is sufficient for room temperature measurements
(Bowey et al. 2001). In the IR and FIR a DTGS/KBr and DTGS/PE detector
in combination with KBr and mylar beamsplitter were used,
respectively. The UV/VIS/NIR and IR spectra were merged together
without an offset or scaling of the curves. A part of the powder
samples has been used for transmission measurements of KBr and PE
pellets in the IR range (2 to 300
m were performed by means of a Bruker FTIR
spectrometer 113v. In this spectral region a gold mirror reference
came into operation. The spectral resolution in the IR was 2 cm-1 which is sufficient for room temperature measurements
(Bowey et al. 2001). In the IR and FIR a DTGS/KBr and DTGS/PE detector
in combination with KBr and mylar beamsplitter were used,
respectively. The UV/VIS/NIR and IR spectra were merged together
without an offset or scaling of the curves. A part of the powder
samples has been used for transmission measurements of KBr and PE
pellets in the IR range (2 to 300 ![]() m). For this purpose,
grains smaller than 1
m). For this purpose,
grains smaller than 1 ![]() m were produced by additional grinding
and sedimentation of the grains in water-free alcohol.
m were produced by additional grinding
and sedimentation of the grains in water-free alcohol.
![\begin{figure}
\par\resizebox{8.8cm}{!}{\includegraphics[clip]{2749_F1.eps}}\end{figure}](/articles/aa/full/2003/34/aa2749/Timg9.gif) |
Figure 1:
IR
transmission spectra of magnesium silicates in the binary system
MgO |
| Open with DEXTER | |
The appearance of a third band at 12.5 ![]() m,
visible in the SiO2 spectrum, is typical for SiO2 and disappears at a
MgO content of 0.5. The band can be assigned to the symmetric
stretching vibration of Si-O-Si bonds or to ring structures (Fuxi 1979; Brinker & Scherer 1990).
The small double band at about 7
m,
visible in the SiO2 spectrum, is typical for SiO2 and disappears at a
MgO content of 0.5. The band can be assigned to the symmetric
stretching vibration of Si-O-Si bonds or to ring structures (Fuxi 1979; Brinker & Scherer 1990).
The small double band at about 7 ![]() m is caused by traces of
magnesium carbonate which can be formed during grinding. In order to separate large
grains (
m is caused by traces of
magnesium carbonate which can be formed during grinding. In order to separate large
grains (![]() 1
1 ![]() m) from the powder sample we used the sedimentation technique
which lead to a slight enrichment of the carbonate in the sample because of the smaller
specific weight of the carbonate grains. By comparison of the integral intensity of
the 7 micron band with selected carbonate samples we could roughly estimate
the carbonate content to 6 mol%. The carbonate impurity was
only found in the ground and sedimented magnesium silicate sample
which was used for the transmission measurement and is not an impurity in the reflection samples.
m) from the powder sample we used the sedimentation technique
which lead to a slight enrichment of the carbonate in the sample because of the smaller
specific weight of the carbonate grains. By comparison of the integral intensity of
the 7 micron band with selected carbonate samples we could roughly estimate
the carbonate content to 6 mol%. The carbonate impurity was
only found in the ground and sedimented magnesium silicate sample
which was used for the transmission measurement and is not an impurity in the reflection samples.
The position of the Si-O stretching vibration is shifted from 9 ![]() m
for the pure SiO2 to 9.7
m
for the pure SiO2 to 9.7 ![]() m for MgSiO3 and 10.25
m for MgSiO3 and 10.25 ![]() m
for the Mg2.4SiO4.4. The influence of the MgO content on the position of the Si-O bending vibration
groups is difficult to observe since the widths of the 20
m
for the Mg2.4SiO4.4. The influence of the MgO content on the position of the Si-O bending vibration
groups is difficult to observe since the widths of the 20 ![]() m bands rise strongly with
increasing MgO content. The position of this feature seems to remain constant. This effect
is related to morphological and matrix effects in the KBr. The KBr
environment leads to a broadening and a long-wavelength shift of both peaks. However, the matrix
influence is much stronger for the very broad 20
m bands rise strongly with
increasing MgO content. The position of this feature seems to remain constant. This effect
is related to morphological and matrix effects in the KBr. The KBr
environment leads to a broadening and a long-wavelength shift of both peaks. However, the matrix
influence is much stronger for the very broad 20 ![]() m bands. We assume that different
bonds within the silicate network such as Si-O and Mg-O will be differently influenced by KBr.
m bands. We assume that different
bonds within the silicate network such as Si-O and Mg-O will be differently influenced by KBr.
![\begin{figure}
\par\resizebox{8.8cm}{!}{\includegraphics[clip]{2749_F2.eps}}\end{figure}](/articles/aa/full/2003/34/aa2749/Timg11.gif) |
Figure 2: Comparison between the calculated reflection spectra (solid lines) and the measured reflection spectra (dashed lines) demonstrated for two silicates. |
| Open with DEXTER | |
Table 2:
Diagnostic spectral parameters of the 10 and 20 ![]() m
bands of amorphous magnesium silicates based on calculations of
the absorption cross section per unit volume
m
bands of amorphous magnesium silicates based on calculations of
the absorption cross section per unit volume
![]() (in cm-1) for small
spheres (Sph.) and a continuous distribution of ellipsoids (CDE)
for particles in the Rayleigh limit.
(in cm-1) for small
spheres (Sph.) and a continuous distribution of ellipsoids (CDE)
for particles in the Rayleigh limit. ![]() and A represent
the peak position in
and A represent
the peak position in ![]() m and the absorption cross section per unit volume
m and the absorption cross section per unit volume
![]() in cm-1, respectively. W stands for the FWHM of the bands.
The band ratio has been calculated by dividing the intensity of
the 20
in cm-1, respectively. W stands for the FWHM of the bands.
The band ratio has been calculated by dividing the intensity of
the 20 ![]() m band by the intensity of the 10
m band by the intensity of the 10 ![]() m band.
m band.
Optical constants (n,k) of the produced stoichiometric and
nonstoichiometric magnesium silicates have been derived from
the reflection measurements (see Fig. 3). For Mg0.7SiO2.7 the
Lorentz-oscillator fit method was exclusively used to determine the complex refractive
index in the whole measurement range from the UV up to the FIR. For the other magnesium
silicates n and k were evaluated by a combination of Kramers-Kronig analysis (KKR) and
Lorentz-oscillator fit method. Concretely, we performed a KKR analysis in the IR range
from 2 to 200 ![]() m. The FIR tail starting from 200
m. The FIR tail starting from 200 ![]() m and the NIR tail starting
from 2
m and the NIR tail starting
from 2 ![]() m were extrapolated assuming that the reflectivities converge to constant values
at zero and infinite wavelengths, respectively. The fulfillment of the causality criterion
m were extrapolated assuming that the reflectivities converge to constant values
at zero and infinite wavelengths, respectively. The fulfillment of the causality criterion
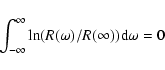 |
In the astronomical literature, the properties of
these very broad oscillator profiles have been rarely addressed
hitherto. However, the optical constants derived for different
amorphous cosmic dust analogs are generally characterized by the
condition
![]() and the corresponding broad
profile (see e.g. Ossenkopf et al. 1992; Eriksson et al. 1981; Draine & Lee 1984; Begemann et al. 1997; Dorschner et al. 1995).
and the corresponding broad
profile (see e.g. Ossenkopf et al. 1992; Eriksson et al. 1981; Draine & Lee 1984; Begemann et al. 1997; Dorschner et al. 1995).
KKR analysis could not be used in the UV/VIS/NIR region, since at
the short wavelength side the spectra end in the region of electronic
transitions, whereas the KKR method requires
a negligible absorption at both spectral limits.
Therefore, the Lorentz-oscillator fit method has been applied to
calculate the n and k values below 14 ![]() m. As for the
applicability of this method, we refer to the monographs by
Wooten (1972) and Bohren & Huffman (1983), who show that in the
UV/VIS range, Lorentz oscillator fits can be used to model all
direct interband transitions in insulators. In order to model the tail of the
electronic transitions in
our magnesium silicates we used 2 oscillators, a broad and very strong one at about
60 000 cm-1 representing the expected strong electronic
transitions below 190 nm and being mainly responsible for the
refractive index in the visible. A second oscillator at about 53 000 cm-1 was used
to model the fine structure of the absorption edge in the range between 210 and 190 nm for
each individual composition of magnesium silicates. The 10
m. As for the
applicability of this method, we refer to the monographs by
Wooten (1972) and Bohren & Huffman (1983), who show that in the
UV/VIS range, Lorentz oscillator fits can be used to model all
direct interband transitions in insulators. In order to model the tail of the
electronic transitions in
our magnesium silicates we used 2 oscillators, a broad and very strong one at about
60 000 cm-1 representing the expected strong electronic
transitions below 190 nm and being mainly responsible for the
refractive index in the visible. A second oscillator at about 53 000 cm-1 was used
to model the fine structure of the absorption edge in the range between 210 and 190 nm for
each individual composition of magnesium silicates. The 10 ![]() m
IR band has been included in the modeling using 2-4 oscillators in
order to achieve an overlap of the results obtained by KKR and
Lorentz-oscillator fitting. It turned out that Lorentz-oscillator
model was better suited to represent the tail of the infrared
bands below 8
m
IR band has been included in the modeling using 2-4 oscillators in
order to achieve an overlap of the results obtained by KKR and
Lorentz-oscillator fitting. It turned out that Lorentz-oscillator
model was better suited to represent the tail of the infrared
bands below 8 ![]() m. Therefore, we merged both results at
this spectral position.
m. Therefore, we merged both results at
this spectral position.
The validity of our calculations is demonstrated by the comparison
between the original reflectance spectra of the silicates and the
calculated reflection determined from the optical constants shown in Fig. 3.
The measured and calculated reflection
curves, displayed in Fig. 2, show a very good coincidence. Only small
deviations in the band region of the Mg0.7SiO2.7 spectrum can be
seen, which result from the
difficulty to reproduce the broad vibrational bands of amorphous
samples with Lorentz-oscillators. Efimov (1999) has compared quantitative analysis
methods of IR spectra of various inorganic glasses to
determine optical constants and found that the Kramers-Kronig
transformation and the Lorentz-oscillator fit method provide practically
coinciding optical constants. KKR can have advantages compared to the
oscillator-fit-method since KKR involves no microphysical model for the
response of solid material to the
electromagnetic field. That means that KKR is not correlated to any
particular band shape, which guarantees the practicability of this
relation to spectra independent of the frequency range and the
materials' properties. Therefore, KKR is a generally accepted method
for the determination of optical constants of solids including amorphous materials
(Kamitsos et al. 1995; Gaskell & Johnson 1976; Aasland et al. 1997; Hudgens & Martin 1996).
![\begin{figure}
\par\resizebox{8.8cm}{!}{\includegraphics[clip]{2749_F3.eps}}\end{figure}](/articles/aa/full/2003/34/aa2749/Timg26.gif) |
Figure 3: Optical constants of amorphous magnesium silicates of varying composition derived from reflection measurements by KKR and Lorentz oscillator fit methods. The n- and k-data files can be taken from the internet homepage of the Astrophysical Institute and University Observatory Jena (http://www.astro.uni-jena.de/Laboratory/Database/silicates.html). |
| Open with DEXTER | |
The knowledge of the optical constants enables us to calculate the
absorption cross section per unit volume of grains with different morphology (see
Table 2 and Fig. 12). The calculated absorption cross sections have
shown that MgO influences the position of the 10 and 20 ![]() m
band. With increasing MgO content, the 10
m
band. With increasing MgO content, the 10 ![]() m band is shifted
to longer wavelengths, whereas the 20
m band is shifted
to longer wavelengths, whereas the 20 ![]() m band is shifted in
the opposite direction. This result is in agreement with the
findings of McMillan (1984), who demonstrated the influence of
alkaline and alkaline earth oxides on the positions of the
stretching and bending Si-O modes in silicate glasses and melts.
The positions of the 10 and 20
m band is shifted in
the opposite direction. This result is in agreement with the
findings of McMillan (1984), who demonstrated the influence of
alkaline and alkaline earth oxides on the positions of the
stretching and bending Si-O modes in silicate glasses and melts.
The positions of the 10 and 20 ![]() m bands are sensitive to the
number of bridging oxygen atoms and therefore to the degree of
polymerization of SiO4 tetrahedra in the amorphous silicate
network. The 10
m bands are sensitive to the
number of bridging oxygen atoms and therefore to the degree of
polymerization of SiO4 tetrahedra in the amorphous silicate
network. The 10 ![]() m bands are broadened due to the
superposition of individual bands of Si-O bonds, whereas the huge increase of the 20
m bands are broadened due to the
superposition of individual bands of Si-O bonds, whereas the huge increase of the 20 ![]() m
band widths' are caused by superposition of Si-O and Mg-O bonds,
varying in bond lengths and angles. Differences to measured transmission
spectra of small grains in KBr, especially in the range of the
20
m
band widths' are caused by superposition of Si-O and Mg-O bonds,
varying in bond lengths and angles. Differences to measured transmission
spectra of small grains in KBr, especially in the range of the
20 ![]() m band, result from matrix and morphological effects in
the KBr pellets.
m band, result from matrix and morphological effects in
the KBr pellets.
![\begin{figure}
\par\resizebox{8.8cm}{!}{\includegraphics[clip]{2749_F4.eps}}\end{figure}](/articles/aa/full/2003/34/aa2749/Timg27.gif) |
Figure 4: Comparison of the optical data of Mg2SiO4 and MgSiO3 produced by the sol-gel method with those of iron-containing proxene- and olivine glasses. |
| Open with DEXTER | |
Figure 4 shows the comparison of our n and k data with optical data of pyroxene and olivine glasses produced by melting and quenching techniques (Dorschner et al. 1995). The spectral behaviour in the UV/VIS range is influenced by the presence of transition metal ions. The incorporation of iron in glasses leads to a series of electronic transitions from the UV up to the NIR range which is well discernible in the iron-containing glasses in Fig. 4. It is well known that in pure magnesium silicate glasses the UV absorption edge is determined by the interaction of UV radiation with electrons of bridging and non-bridging oxygen. The strong and rather broad absorptions at wavelengths smaller than 200 nm are caused by electronic transitions, concretely, by excitons and interband transitions between the 2p orbitals of oxygen and the 3d orbitals of silicon (Fuxi 1979; Liepmann & Neuroth 1995). Since the non-bridging oxygen are less tightly bound to silicon than the bridging oxygens their electronic transitions occur at longer wavelengths than those of bridging oxygen.
![\begin{figure}
\par\resizebox{8.8cm}{!}{\includegraphics[clip]{2749_F5.eps}}\end{figure}](/articles/aa/full/2003/34/aa2749/Timg28.gif) |
Figure 5: Comparison of the optical constants of amorphous Mg2SiO4 produced by sol-gel method (solid line) and optical data of Mg2SiO4 films (dotted line) derived by Scott & Duley (1996). |
| Open with DEXTER | |
Table 3:
Activation energies of crystallization
![]() determined for differently produced magnesium silicates.
determined for differently produced magnesium silicates.
One can draw the conclusion that just as in the case of amorphous carbon, different structures with varying optical constants may exist and that the short-range as well as the medium-range order determine their optical properties in the UV/VIS and in the IR region.
![\begin{figure}
\par\resizebox{8.8cm}{!}{\includegraphics[clip]{2749_F6.eps}}\end{figure}](/articles/aa/full/2003/34/aa2749/Timg30.gif) |
Figure 6: Comparison of the absorption cross section of amorphous Mg2SiO4 produced by sol-gel method with that of Mg2SiO4 films calculated from the n and k data by Scott & Duley (1996). The solid lines stand for spherical particles, whereas the dotted lines represent the results for a continuous distribution of ellipsoids. The cross sections derived from our data have been offset by a factor of 10. |
| Open with DEXTER | |
In this paper we use an empirical approach for the description of the
crystallization enclosing both processes. Infrared spectroscopy
has been used for monitoring the progress of annealing. The
characteristic annealing time ![]() is determined by the
appearance of first hints of fine structure indicating the
increase of structural order. The activation energy of
crystallization for the pure sol-gel produced magnesium silicates
has been calculated using the equation
is determined by the
appearance of first hints of fine structure indicating the
increase of structural order. The activation energy of
crystallization for the pure sol-gel produced magnesium silicates
has been calculated using the equation
![]() ,
wherein
,
wherein ![]() is the activation energy and
is the activation energy and
![]() a constant proportional to the mean vibrational frequency
of the silicate lattice (
a constant proportional to the mean vibrational frequency
of the silicate lattice (
![]() s-1; Lenzuni et al. 1995; Gail & Sedlmayr 1998).
s-1; Lenzuni et al. 1995; Gail & Sedlmayr 1998).
The results of the annealing experiments are summarized in Table 3. For the sake of comparison, the table additionally
contains activation energies of differently produced silicate
materials like glasses and smokes (Fabian et al. 2000). The
activation energies
![]() of magnesium silicates produced by
sol-gel synthesis amount to values between 26 600 and 31 700 K
corresponding to about two thirds of the crystallization
activation energy of Mg-silicate glasses and smokes. Corresponding
annealing temperatures were between 723 and 873 K. Annealing
experiments with sol-gel produced magnesium silicates demonstrate
that
of magnesium silicates produced by
sol-gel synthesis amount to values between 26 600 and 31 700 K
corresponding to about two thirds of the crystallization
activation energy of Mg-silicate glasses and smokes. Corresponding
annealing temperatures were between 723 and 873 K. Annealing
experiments with sol-gel produced magnesium silicates demonstrate
that ![]() is independent of the composition of the materials.
This unusual behaviour has its seeds in a content of
non-associated Si-OH and Mg-OH bonds stemming from the
sol-gel synthesis.
is independent of the composition of the materials.
This unusual behaviour has its seeds in a content of
non-associated Si-OH and Mg-OH bonds stemming from the
sol-gel synthesis.
Non-associated Si-OH and Mg-OH bonds give rise to a narrow
band at about 2.7 ![]() m due to O-H stretching vibrations (see
Fig. 7). These groups can remain from the condensation
process. However, the band position of 3670 cm-1 rather
points to an excess of Si-OH bonds (Morimoto et al. 1999; Iler 1979a)
compared to Mg-OH since the latter have a band at 3700 cm-1(Shinoda et al. 2002; Mitra 1962). In case of reduced temperature
crystallization this narrow band at 2.7
m due to O-H stretching vibrations (see
Fig. 7). These groups can remain from the condensation
process. However, the band position of 3670 cm-1 rather
points to an excess of Si-OH bonds (Morimoto et al. 1999; Iler 1979a)
compared to Mg-OH since the latter have a band at 3700 cm-1(Shinoda et al. 2002; Mitra 1962). In case of reduced temperature
crystallization this narrow band at 2.7 ![]() m has been found in
addition to the first spectroscopic hints to crystallization due
to a content of non-associated O-H bonds in these samples. The
content of these O-H bonds has been spectroscopically determined
by comparison of the 2.7
m has been found in
addition to the first spectroscopic hints to crystallization due
to a content of non-associated O-H bonds in these samples. The
content of these O-H bonds has been spectroscopically determined
by comparison of the 2.7 ![]() m band with a sample of well known
Si-OH content. The latter does not exceed 1.3 wt% that remained
from the sol-gel synthesis. Isolated O-H bonds in a
magnesium silicate framework reduce the formation of oxygen
bridges between the SiO4 tetrahedra and form non-bridging
oxygen. The increase of non-bridging oxygen leads to a decrease of
the viscosity (Scholze 1988). The influence of OH groups is
comparable to that of alkaline ions which act as network modifier
introducing non-bridging oxygen (Iler 1979b). This is easy to
understand since the proton H+ of the OH group can be
considered like a alkaline ion. Thereby the viscosity of the
magnesium silicates is decreased and the crystallization
temperature can be lowered by 200 to 300 K (cf. Fig. 7). That means that the crystallization temperature is
dependent on the Si-OH content. A further incorporation of Si-OH
does not lead to a further decrease of the crystallization
temperature. The temperature of crystallization can be decreased
from 1000 K for Si-OH free material to 730 K for magnesium
silicate containing 1.3 wt% Si-OH bonds.
m band with a sample of well known
Si-OH content. The latter does not exceed 1.3 wt% that remained
from the sol-gel synthesis. Isolated O-H bonds in a
magnesium silicate framework reduce the formation of oxygen
bridges between the SiO4 tetrahedra and form non-bridging
oxygen. The increase of non-bridging oxygen leads to a decrease of
the viscosity (Scholze 1988). The influence of OH groups is
comparable to that of alkaline ions which act as network modifier
introducing non-bridging oxygen (Iler 1979b). This is easy to
understand since the proton H+ of the OH group can be
considered like a alkaline ion. Thereby the viscosity of the
magnesium silicates is decreased and the crystallization
temperature can be lowered by 200 to 300 K (cf. Fig. 7). That means that the crystallization temperature is
dependent on the Si-OH content. A further incorporation of Si-OH
does not lead to a further decrease of the crystallization
temperature. The temperature of crystallization can be decreased
from 1000 K for Si-OH free material to 730 K for magnesium
silicate containing 1.3 wt% Si-OH bonds.
A similar effect can be observed in the case of Fe incorporation.
The incorporation of only 5 Mol% Fe reduces the activation energy
![]() by about 2000-3000 K in comparison with the iron-free
magnesium silicate. This is due to the decrease of viscosity
beyond the transformation range of the amorphous material. The
calculation of the activation energy enables us to compare the
crystallization behaviour of different materials and to estimate
time scales necessary for crystallization at different
temperatures. For example, a magnesium silicate without Si-OH
groups needs
by about 2000-3000 K in comparison with the iron-free
magnesium silicate. This is due to the decrease of viscosity
beyond the transformation range of the amorphous material. The
calculation of the activation energy enables us to compare the
crystallization behaviour of different materials and to estimate
time scales necessary for crystallization at different
temperatures. For example, a magnesium silicate without Si-OH
groups needs ![]() yrs for crystallization at a
temperature of 750 K, whereas Si-OH-containing
magnesium silicates crystallize in minutes up to hours at this
temperature. This time scale matches well to the expected
residence times of condensed silicates close to the condensation
zone. The formation of Si-OH bonds in cosmic magnesium silicate
particles has to be taken into account since H2O as the most
abundant oxygen-bearing molecule plays a very important role in
the condensation of circumstellar silicates (Gail & Sedlmayr 1998).
yrs for crystallization at a
temperature of 750 K, whereas Si-OH-containing
magnesium silicates crystallize in minutes up to hours at this
temperature. This time scale matches well to the expected
residence times of condensed silicates close to the condensation
zone. The formation of Si-OH bonds in cosmic magnesium silicate
particles has to be taken into account since H2O as the most
abundant oxygen-bearing molecule plays a very important role in
the condensation of circumstellar silicates (Gail & Sedlmayr 1998).
![\begin{figure}
\par\resizebox{8.45cm}{!}{\includegraphics[clip]{2749_F7.eps}}\end{figure}](/articles/aa/full/2003/34/aa2749/Timg37.gif) |
Figure 7:
Powder transmission spectra of Mg2SiO4 (in KBr
pellets) with varying Si-OH content. All the samples have been
annealed for 1h. The spectra clearly show the correlation between
crystallization and the appearance of the characteristic
vibrational band of isolated Si-OH bonds located at 2.7 |
| Open with DEXTER | |
For the comparison with the observations we chose the two AGB stars
TY Draconis and R Cassiopeiae,
for which high-quality ISO-SWS spectra in the wavelength range
from 2 to 45 ![]() m are available. These spectra have been
downloaded from the ISO archive and were reduced with the ISO
Spectral Analysis Package (ISAP). TY Dra shows very slender bands
and a deep trough, whereas the Mira star R Cas represents the case
of very broad bands and a shallow trough.
As effective dust temperature we adopted 400 K, that
corresponds to the values derived in the literature for
Miras and semi-regular variables (Hron et al. 1997).
m are available. These spectra have been
downloaded from the ISO archive and were reduced with the ISO
Spectral Analysis Package (ISAP). TY Dra shows very slender bands
and a deep trough, whereas the Mira star R Cas represents the case
of very broad bands and a shallow trough.
As effective dust temperature we adopted 400 K, that
corresponds to the values derived in the literature for
Miras and semi-regular variables (Hron et al. 1997).
![\begin{figure}
\par\resizebox{8.8cm}{!}{\includegraphics[clip]{2749_F8.eps}}\end{figure}](/articles/aa/full/2003/34/aa2749/Timg38.gif) |
Figure 8: Comparison of our sol-gel-produced magnesium silicates with the observed ISO-SWS spectrum of TY Draconis. In the second line of the insert the full silicate formulae have been truncated in order to save space. The mixture of Mg1.5SiO3.5, Mg2SiO4, and Mg2.4SiO4.4 (for a CDE) gives the best reproduction of the dust emissivity reached so far. |
| Open with DEXTER | |
The spectra of R Cas and TY Dra have been used for this purpose, not only because of the characteristic difference in the MIR spectra of both stars, but also because of the fact that for both of them, several ISO spectra exist, dating from different phases in their cycles of variability. The different spectra of R Cas strongly resemble each other in the region dominated by the dust emission (Kerschbaum et al. 2000), and the same is true for the spectra of TY Dra. This indicates that intrinsic differences between both stars are responsible for their largely different MIR dust emission.
The dust emissivity has been derived in the following way: At
![]() m, the observed flux was assumed to be wholly
dominated by the stellar photosphere. Therefore, a Rayleigh
function has been fitted to the spectral energy distribution
at this wavelength and has been subtracted from the SED in order
to derive the dust emissivity. Subtracting more sophisticated
photospheric models which take into account the molecular
absorption (primarily due to H2O, CO and SiO) does not lead
to significantly different results for the dust emissivity in this case,
especially not for the region
m, the observed flux was assumed to be wholly
dominated by the stellar photosphere. Therefore, a Rayleigh
function has been fitted to the spectral energy distribution
at this wavelength and has been subtracted from the SED in order
to derive the dust emissivity. Subtracting more sophisticated
photospheric models which take into account the molecular
absorption (primarily due to H2O, CO and SiO) does not lead
to significantly different results for the dust emissivity in this case,
especially not for the region ![]() > 9
> 9 ![]() m in which we
are primarily interested. It is clear from
radiative transfer calculations that for stars like TY Dra, the
contribution of the dust emission to the total SED amounts to already
5-10% in the 7
m in which we
are primarily interested. It is clear from
radiative transfer calculations that for stars like TY Dra, the
contribution of the dust emission to the total SED amounts to already
5-10% in the 7 ![]() m region. Therefore, the subtraction
procedure explained above introduces a small uncertainty of the
dust emissivity in the 7-9
m region. Therefore, the subtraction
procedure explained above introduces a small uncertainty of the
dust emissivity in the 7-9 ![]() m range. In Fig. 8, the normalized dust spectrum of TY Dra obtained
by the described procedure is compared with emissivities
determined from the calculated absorption coefficients of the sol-gel silicates.
While the observed spectrum cannot be satisfactorily reproduced by
using a single magnesium silicate compound, a mixture of
Mg1.5SiO3.5, Mg2SiO4 and Mg2.4SiO4.4gives an almost perfect agreement with the observation. This shows that pure amorphous
magnesium silicates are the best candidates for the dust spectra of such stars.
The biggest deviation is visible at the "blue'' wing of the 10
m range. In Fig. 8, the normalized dust spectrum of TY Dra obtained
by the described procedure is compared with emissivities
determined from the calculated absorption coefficients of the sol-gel silicates.
While the observed spectrum cannot be satisfactorily reproduced by
using a single magnesium silicate compound, a mixture of
Mg1.5SiO3.5, Mg2SiO4 and Mg2.4SiO4.4gives an almost perfect agreement with the observation. This shows that pure amorphous
magnesium silicates are the best candidates for the dust spectra of such stars.
The biggest deviation is visible at the "blue'' wing of the 10 ![]() m band.
The corresponding dust emissivity of R Cas (see Fig. 11)
cannot be exclusively represented by sol-gel magnesium silicates or
by the glassy Mg-Fe-silicates. This is mainly due to R Cas'
enhanced emission in the 11-15
m band.
The corresponding dust emissivity of R Cas (see Fig. 11)
cannot be exclusively represented by sol-gel magnesium silicates or
by the glassy Mg-Fe-silicates. This is mainly due to R Cas'
enhanced emission in the 11-15 ![]() m range, which is
discussed below.
m range, which is
discussed below.
One problem with the magnesium silicates is their very low NIR
opacity, which inhibits effective absorption of the stellar radiation
and therefore strongly reduces heating. However, taking into account the
probable formation of metallic iron in oxygen-rich circumstellar shells
(see e.g. Kozasa & Hasegawa 1988; Gail & Sedlmayr 1999), this problem can be easily solved,
because metallic iron inclusions will strongly
enhance the NIR absorption of Mg silicates as recently demonstrated by Kemper et al. (2002).
Near 1 ![]() m, where the
opacity of pure magnesium silicates reaches its minimum, the value
of
m, where the
opacity of pure magnesium silicates reaches its minimum, the value
of
![]() /a is increased by a factor of about 10 if only 0.1%
of the grain volume is a metallic Fe inclusion. An enhancement by a
factor of 100 is reached if 1% of the grain volume is metallic Fe
(see Fig. 9).
/a is increased by a factor of about 10 if only 0.1%
of the grain volume is a metallic Fe inclusion. An enhancement by a
factor of 100 is reached if 1% of the grain volume is metallic Fe
(see Fig. 9).
![\begin{figure}
\par\resizebox{8.8cm}{!}{\includegraphics[clip]{2749_F9.eps}}\end{figure}](/articles/aa/full/2003/34/aa2749/Timg41.gif) |
Figure 9:
The effect of metallic iron impurities on the absorption
efficiency
|
| Open with DEXTER | |
![\begin{figure}
\par\resizebox{8.8cm}{!}{\includegraphics[clip]{2749_F10.eps}}\end{figure}](/articles/aa/full/2003/34/aa2749/Timg42.gif) |
Figure 10: Reproduction of the mean spectra of AGB star groups A, B, C and supergiants group 1 (Speck et al. 2000) with sol-gel magnesium silicates of different stoichiometry. The solid lines represent the star spectra and the dashed or dotted lines stand for either single or mixtures of magnesium silicates. The AGB stars group C profile (lowest panel) equal portions of all magnesium silicates have been mixed. |
| Open with DEXTER | |
It is a well-known fact that the silicate spectra of AGB-stars and
other evolved stars exhibit great spectral diversity especially in
the 10 ![]() m region. Speck et al. (2000) have used UKIRT CGS3
spectra in order to classify the 10
m region. Speck et al. (2000) have used UKIRT CGS3
spectra in order to classify the 10 ![]() m profiles of AGB stars
and supergiants into characteristic groups. We examined whether
our magnesium silicates are able to match the mean spectra of
selected star groups. Figure 10 illustrates that the
derived mean profiles of group A to C and the additional
supergiant group 1 can be well reproduced either by mixtures of or
single magnesium silicates. The mean spectrum of supergiant
silicate group 1 can be matched by Mg1.5SiO3.5 spheres
in the Rayleigh limit. The spectrum of group A of the AGB stars
can be fitted by either a single magnesium silicate (Mg1.5SiO3.5) or a mixture of the two components
Mg2SiO4 and Mg2.4SiO4.4. In contrast groups C and
D require a rather complex mixture with varying Mg/Si ratios.
m profiles of AGB stars
and supergiants into characteristic groups. We examined whether
our magnesium silicates are able to match the mean spectra of
selected star groups. Figure 10 illustrates that the
derived mean profiles of group A to C and the additional
supergiant group 1 can be well reproduced either by mixtures of or
single magnesium silicates. The mean spectrum of supergiant
silicate group 1 can be matched by Mg1.5SiO3.5 spheres
in the Rayleigh limit. The spectrum of group A of the AGB stars
can be fitted by either a single magnesium silicate (Mg1.5SiO3.5) or a mixture of the two components
Mg2SiO4 and Mg2.4SiO4.4. In contrast groups C and
D require a rather complex mixture with varying Mg/Si ratios.
It turned out that "filling of the trough'' in order to obtain
spectra like that of R Cas cannot be reached by silicate
absorption. Silicates containing alkaline earth or transition metal oxides
cannot provide spectra with 10 ![]() m profiles shifted to wavelengths longer than
11
m profiles shifted to wavelengths longer than
11 ![]() m since the position of this Si-O stretching band depends on the
polymerization degree of SiO4 tetrahedra and therefore on the number of
bridging oxygen atoms in the silicate network (McMillan 1984).
Even though in Mg2SiO4 only non-bridging oxygen atoms are present,
the formation of a three-dimensional amorphous network requires the
existence of at least a small portion of bridging oxygen atoms. This can only be
compensated by the incorporation of structural inhomogeneity on an atomic scale.
A considerably higher content of metal oxide in the silicate gives rise to the
formation of rather inhomogeneous material due to the stoichiometry problems.
In this study we were able to incorporate a metal oxide content up to 2.4 wt%
without producing a non-homogeneous material.
m since the position of this Si-O stretching band depends on the
polymerization degree of SiO4 tetrahedra and therefore on the number of
bridging oxygen atoms in the silicate network (McMillan 1984).
Even though in Mg2SiO4 only non-bridging oxygen atoms are present,
the formation of a three-dimensional amorphous network requires the
existence of at least a small portion of bridging oxygen atoms. This can only be
compensated by the incorporation of structural inhomogeneity on an atomic scale.
A considerably higher content of metal oxide in the silicate gives rise to the
formation of rather inhomogeneous material due to the stoichiometry problems.
In this study we were able to incorporate a metal oxide content up to 2.4 wt%
without producing a non-homogeneous material.
There are, however, oxides that could contribute to
the trough opacity. First observational evidence pointing in this
direction was given by the 13 ![]() m band that has been discussed
as being due to Al-O vibrations in
m band that has been discussed
as being due to Al-O vibrations in ![]() -Al2O3 or in MgAl2O4(Posch et al. 1999; Begemann et al. 1997; Fabian et al. 2001). Further experiments with
other oxides that could effectively contribute to the trough
opacity are in progress in the Jena laboratory.
-Al2O3 or in MgAl2O4(Posch et al. 1999; Begemann et al. 1997; Fabian et al. 2001). Further experiments with
other oxides that could effectively contribute to the trough
opacity are in progress in the Jena laboratory.
In order to roughly illustrate the amount and the cumulative SED of
potential trough opacity contributors from the observations, we simply
subtracted the normalized dust emission of a star like TY Dra from the normalized dust emission
of a star that does not show a deep trough between the two silicate
bands such as R Cas. The result of such a subtraction is shown in Fig. 11.
As already mentioned, this difference spectrum can hardly be understood in
terms of different silicate dust populations. Different grain sizes
could play a role, since for large grains (i.e. when their radius
is no longer small compared to the wavelength), both the 10 and the
20 ![]() m bands can develop huge shoulders partly filling the trough
between them. However, the dominance of grains with diameters of
several
m bands can develop huge shoulders partly filling the trough
between them. However, the dominance of grains with diameters of
several ![]() m is not very probable in an average circumstellar
shell. An exception are very evolved AGB or massive stars, which
can have grain sizes up to 6
m is not very probable in an average circumstellar
shell. An exception are very evolved AGB or massive stars, which
can have grain sizes up to 6 ![]() m (Hoogzaad et al. 2002; Molster et al. 1999).
Optical depth effects are negligible, because the circumstellar
shells of both stars are optically thin over the whole wavelength range
considered here. Therefore, it seems rather likely that a distinct dust
grain population is responsible for the broad emission peaking around
13
m (Hoogzaad et al. 2002; Molster et al. 1999).
Optical depth effects are negligible, because the circumstellar
shells of both stars are optically thin over the whole wavelength range
considered here. Therefore, it seems rather likely that a distinct dust
grain population is responsible for the broad emission peaking around
13 ![]() m. As potential carriers of this emission oxides like
Al2O3 (Begemann et al. 1997), MgAl2O4 (Posch et al. 1999; Fabian et al. 2001) and
Ca-Al-oxides (Mutschke et al. 2002) have been discussed. It is an
interesting coincidence that the only band that has been
definitely observed is located exactly at the 13
m. As potential carriers of this emission oxides like
Al2O3 (Begemann et al. 1997), MgAl2O4 (Posch et al. 1999; Fabian et al. 2001) and
Ca-Al-oxides (Mutschke et al. 2002) have been discussed. It is an
interesting coincidence that the only band that has been
definitely observed is located exactly at the 13 ![]() m peak of the
difference spectrum.
m peak of the
difference spectrum.
![\begin{figure}
\par\resizebox{8.8cm}{!}{\includegraphics[clip]{2749_F11.eps}}\end{figure}](/articles/aa/full/2003/34/aa2749/Timg44.gif) |
Figure 11:
Difference between the normalized dust emissivities of R
Cas and TY Dra. The broad maximum around 13 |
| Open with DEXTER | |
![\begin{figure}
\par\resizebox{8.8cm}{!}{\includegraphics[clip]{2749_F12.eps}}\end{figure}](/articles/aa/full/2003/34/aa2749/Timg45.gif) |
Figure 12: Calculated absorption cross sections per unit volume of the magnesium silicates for spheres (solid lines) and CDE (dashed lines). |
| Open with DEXTER | |
By means of the sol-gel technique, we were able to produce amorphous
magnesium silicates with different Mg/Si ratios in a homogeneous form and to
derive optical constants by Kramers-Kronig analysis and Lorentz-oscillator fit method.
The diagnostic spectral parameters in the MIR region have
been derived by calculating the absorption efficiency in vacuum both for
spheres and for a distribution of ellipsoids in the Rayleigh limit.
The 10 and the 20 ![]() m bands have been
shown to approach each other with increasing magnesium content (which
shifts the 10
m bands have been
shown to approach each other with increasing magnesium content (which
shifts the 10 ![]() m band towards the "red'' and the 20
m band towards the "red'' and the 20 ![]() m band
towards the "blue''). This effect is due to the influence of the MgO content
on the degree of polymerization of the SiO4 tetrahedra. As expected,
large differences between pure
magnesium- and composite magnesium-iron-silicates were found in the
NIR region.
m band
towards the "blue''). This effect is due to the influence of the MgO content
on the degree of polymerization of the SiO4 tetrahedra. As expected,
large differences between pure
magnesium- and composite magnesium-iron-silicates were found in the
NIR region.
The crystallization of amorphous Mg-silicates by thermal annealing has also been investigated. The sol-gel produced magnesium silicates show an interesting annealing behaviour. The activation energy was found to be independent of the Mg/Si ratio, but largely dependent on the Si-OH content. The activation energy of crystallization can be reduced by one third by the presence of up to 1.5 wt% Si-OH bonds compared to magnesium silicates which do not contain Si-OH. This leads to a significant reduction of the necessary crystallization temperature and crystallization time.
First results of a comparison with observed dust emission spectra underline the relevance of amorphous Mg-silicates for our understanding of circumstellar shells. Comparisons with the normalized dust spectrum of TY Dra have demonstrated that a mixture of Mg1.5SiO3.5, Mg2SiO4 and Mg2.4SiO4.4gives a nearly perfect match with the observations. Pure amorphous magnesium silicates are the best candidates for modeling the dust spectra of such stars.
The corresponding dust emissivity of R Cas (see Fig. 11)
cannot be exclusively represented by sol-gel magnesium silicates or
by the glassy Mg-Fe-silicates.
Other dust components like aluminium oxides or spinels can contribute to
the trough opacity. The trough opacity
beyond 16 ![]() m could be influenced by iron oxide or mixed magnesium
iron oxides. Furthermore, different grain sizes could play a role,
since for large grains (i.e. when their radius
is no longer small compared to the wavelength), both the 10 and the
20
m could be influenced by iron oxide or mixed magnesium
iron oxides. Furthermore, different grain sizes could play a role,
since for large grains (i.e. when their radius
is no longer small compared to the wavelength), both the 10 and the
20 ![]() m bands can develop huge shoulders partly filling the trough
between them. However, the dominance of grains with diameters of
several
m bands can develop huge shoulders partly filling the trough
between them. However, the dominance of grains with diameters of
several ![]() m is not very probable in an average circumstellar
shell.
m is not very probable in an average circumstellar
shell.
Acknowledgements
This work has been supported by the Deutsche Forschungsgemeinschaft (DFG grant DO 575/2-3). TP received a DOC grant by the Austrian Academy of Sciences. We gratefully thank A.K. Speck for providing us 10m spectra of oxygen-rich evolved stars for comparison. Furthermore, we would like to thank G. Born for her help in the laboratory.