- ... capabilities
![[*]](/icons/foot_motif.gif)
- We note that chemical non-equilibrium effects
in the time-independent case may even lead to spurious effects
since existing chemical rate networks do usually not lead to the
chemical equilibrium solution for
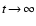 ,
even if
,
even if
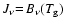 is assumed, which contradicts very
basic thermodynamical considerations. The reason is that usually
the corresponding forward and reverse processes are either not
included pair-wise in all cases or that the respective rate
coefficients are not properly related to each other via the Gibb's
free energies of the molecules, but rather treated in an
independent way.
is assumed, which contradicts very
basic thermodynamical considerations. The reason is that usually
the corresponding forward and reverse processes are either not
included pair-wise in all cases or that the respective rate
coefficients are not properly related to each other via the Gibb's
free energies of the molecules, but rather treated in an
independent way.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ... case
![[*]](/icons/foot_motif.gif)
- Other, eventually multi-parametric descriptions of
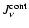 are also conceivable, e.g. adjusted to the
optically thin case.
are also conceivable, e.g. adjusted to the
optically thin case.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ... to H atoms
![[*]](/icons/foot_motif.gif)
- In this paper, we rely on the mean molecular weight
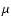 as indicator for the dissociative state of hydrogen, because
in our dynamical calculations, the state functions enter in a
tabulated form where the actual particle densities
as indicator for the dissociative state of hydrogen, because
in our dynamical calculations, the state functions enter in a
tabulated form where the actual particle densities 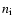 are
"forgotten''.
are
"forgotten''.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ... zones
![[*]](/icons/foot_motif.gif)
- Actually no single zones are split, but three new
zones are carefully constructed out of two old ones, thus preventing
numerical generation of mass or momentum.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ... since
![[*]](/icons/foot_motif.gif)
- Neglecting the difference between
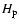 and
and 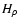 .
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ... functions
![[*]](/icons/foot_motif.gif)
- A detailed statistical comparison
between new and classical models was not the aim of this work, but
might be presented in a subsequent paper.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ... parameters
![[*]](/icons/foot_motif.gif)
- The choice of
these parameters was guided by investigations of (Richter et al. 2002) on Fe II
emission lines.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ...
![[*]](/icons/foot_motif.gif)
- See also
http://astro.physik.tu-berlin.de/~woitke/Mira.mpeg.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ... work
![[*]](/icons/foot_motif.gif)
- Referred to as "adiabatical heating'',
therefore
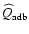 .
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ... case
![[*]](/icons/foot_motif.gif)
- The width of the
complete radiative relaxation zone is smaller than the
grid spacing in this case, resulting in
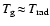 even at points
where
even at points
where
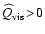 ,
e.g. at the positions of the shock waves.
,
e.g. at the positions of the shock waves.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ... heated/cooled)
![[*]](/icons/foot_motif.gif)
- The internal
chemical processes responsible for the formation of H2 are assumed to be
very fast in our model (assumption of chemical equilibrium, see
Sect. 3.1). Thereby, the actual speed of the H2-formation is
limited by the need of the gas to radiate away the liberated binding energy
in our model. It remains an open question whether the three-body gas phase
reactions and the surface reactions on dust grains required for the
kinetical formation of H2 are actually fast enough to achieve this
limiting case. A kinetic treatment of the H2-formation would be
desirable, but goes beyond the scope of this paper.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ...
gas
![[*]](/icons/foot_motif.gif)
- Strictly speaking, this is not the post-shock-cooling
here. What we observe is rather a quick decrease of
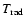 due to
the decreasing backwarming by the outward propagating and thereby
radially diluting dust-shell.
due to
the decreasing backwarming by the outward propagating and thereby
radially diluting dust-shell.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ... environment
![[*]](/icons/foot_motif.gif)
- The shock wave velocity is rather
controlled by
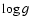 and P than by
and P than by 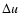 ,
compare
footenote 2 in Paper II.
,
compare
footenote 2 in Paper II.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ... numerics
![[*]](/icons/foot_motif.gif)
- Neither a variation of the
resolution, nor of the averaging procedure
for the velocity gradient
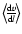 resulted in a qualitative change of this
cooling behaviour. Of course, we
cannot exclude an influence of the numerical method on these
results. However, even if the initial temperature
pertubations are of numerical origin, the amplification
mechanism clearly has a physical nature.
resulted in a qualitative change of this
cooling behaviour. Of course, we
cannot exclude an influence of the numerical method on these
results. However, even if the initial temperature
pertubations are of numerical origin, the amplification
mechanism clearly has a physical nature.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.