A&A 390, 793-800 (2002)
DOI: 10.1051/0004-6361:20020800
Laboratoire d'Astrophysique, UMR CNRS 6091,
Observatoire de Besançon, Université de Franche-Comté,
41 bis avenue de l'Observatoire, BP 1615, 25010 Besançon Cedex, France
Received 25 March 2002 / Accepted 24 May 2002
Abstract
Close coupling (CC) calculations of pure rotational excitation rates of H2O
by para-H2 are performed between 5 K and 20 K. Some discrepancies
with Phillips et al. (1995, 1996) are found and explained. We find that
the presence of resonances greatly influences the excitation rates at
very low temperature. Fits of our collisional rates are provided.
Key words: molecular data - molecular processes
This paper is the first part of a thorough theoretical study of the collisional excitation rates of H2O by H2, which will be used to interpret data from HIFI (Herschel Mission). Many transitions of the H2O molecule will be observed by HIFI in different environments such as the interstellar medium and stellar or planetary atmospheres. These observations will complete the wealth of data already obtained by the Infrared Space Observatory (see for example Spinoglio et al. 2001; Tsuji 2001; Wright et al. 2000), and the Submillimeter Wave Astronomy Satellite (Melnick et al. 2000). Collisional rotational and ro-vibrational excitation rates of H2O by H2 are essential for the interpretation of excitation conditions and for the determination of chemical composition in the different media.
The only available excitation rates of H2O by H2 are those calculated by Phillips et al. (1995, 1996), using the close-coupling CC and the coupled states (CS) methods with a potential energy surface (PES) calculated by Phillips et al. (1994). Those authors provided data between 20 K and 140 K for a number of ortho/para H2O - ortho/para H2 pure rotational transitions. A previous study by Green et al. (1993) provided the excitation rotational rates of H2O by He for temperature between 20 K and 2000 K using the improved PES of Maluendes et al. (1992).
The main objective of the present paper is the determination of pure rotational
excitation rates of ortho/para H2O by para H2,
at very low temperatures (![]() K) where no
data have yet been calculated. We use the same PES (Phillips et al. 1994) and
the same description of the water molecule as Phillips et al. (1995),
which allows us to compare the collision rates we obtain at 20 K with those of
Phillips et al. (1995, 1996). Furthermore we examine the validity of a
fitted function that is sometimes used in the interpretation of observed
spectra or in modelling the ISM.
K) where no
data have yet been calculated. We use the same PES (Phillips et al. 1994) and
the same description of the water molecule as Phillips et al. (1995),
which allows us to compare the collision rates we obtain at 20 K with those of
Phillips et al. (1995, 1996). Furthermore we examine the validity of a
fitted function that is sometimes used in the interpretation of observed
spectra or in modelling the ISM.
Both the description of the quantum calculations and the convergence of the calculated collisional cross sections are presented in Sect. 2. The analysis of the rotational excitation rates is considered in Sect. 3.
Close coupling and coupled states calculations are done with the
MOLSCAT (Hutson & Green 1994) code and with Green's code of
the PES of Phillips et al. (1994). The water molecule is described by
a version of the effective Hamiltonian of Kyrö (1981),
compatible with the symmetries of the PES.
We use the molecular constants
of Table 1 of Kyrö (1981) and our calculated rotational
levels of H216O are identical to those of Green et al. (1993).
The reduced mass
of the system is 1.81277373 a.m.u. and the hydrogen molecule is
taken as a rigid rotor with a rotational constant of 60.853 cm-1 .
| Propagation parameters | ||
| INTFLG = 6 | STEPS = 10 | |
| DTOL = 0.3 | OTOL = 0.005 | |
| (1)RMIN = 1.2 (adjusted) | (1)IRMSET = 10 | (1)RMAX = 40. (adjusted) |
| (2)RMIN = 1. (adjusted) | (2)IRMSET = 0 | (2)RMAX = 50. (adjusted) |
| Basis set B(j, j2) | ||
| 1 closed j-channel for H20 | ||
| 1 or 0 closed j2-channel for H2 | (as indicated in the text) |
Our first step was to assess the correctness of our results
with respect to those of Phillips et al. (1995). We re-calculated
the inelastic cross sections for all symetries and at the energies given
in Tables 1 and 2 of Phillips et al. (1995).
Most of our inelastic
cross sections, calculated with the parameters values given in
Table 1, are
in excellent agreement with those of Phillips et al. (1995).
Table 2 shows the few
CC cross sections that do not agree very well.
| Energy | Initial | Final | Our values | Phillips's | Relative difference |
| para-H2O/para-H2 | |||||
| 47 | 00,0 | 11,1 | 2.85 | 2.91 | 2% |
| 300 | 32,2 | 33,1 | 0.73 | 0.71 | 2.8% |
| ortho-H2O/para-H2 | |||||
| 123.79 | 10,1 | 21,2 | 1.99 | 1.94 | 2.6% |
| 300 | 31,2 | 32,1 | 1.13 | 1.10 | 2.7% |
| 32,1 | 33,0 | 0.80 | 0.77 | 3.9% | |
| 33,0 | 41,4 | 0.52 | 0.49 | 6.1% |
We tested the convergence of the results with respect to the parameters
in Table 1, for the two propagators used by
Phillips et al. (1995, 1996), namely the
diabatic modified log-derivative method of Manolopoulos (1986)
(parameter
![]() in MOLSCAT) and the hydrid propagator of
Alexander & Manolopoulos (1987) (parameter
in MOLSCAT) and the hydrid propagator of
Alexander & Manolopoulos (1987) (parameter
![]() in MOLSCAT).
We tested these propagators both with and without an automatic search for the
starting point, meaning that the parameter IRMSET is either
varied until convergence is obtained or set to zero.
This gives 4 different methods for each of CC and CS calculations.
For both propagators the parameters to be optimized are the
number of steps per half wave number at the lowest energy (STEPS),
the maximum range of propagation (RMAX) and either
the starting point of propagation (RMIN) if it is set fixed or the
initial wavefunction amplitude in all channels (less than 10**(-IRMSET)).
For the hybrid propagator additional parameters
(RMID or RVFAC, DRAIRY), managing
the transition between the short and the long range propagations, must be
optimized.
We performed the convergence tests of the CC and CS
cross sections of the transition
in MOLSCAT).
We tested these propagators both with and without an automatic search for the
starting point, meaning that the parameter IRMSET is either
varied until convergence is obtained or set to zero.
This gives 4 different methods for each of CC and CS calculations.
For both propagators the parameters to be optimized are the
number of steps per half wave number at the lowest energy (STEPS),
the maximum range of propagation (RMAX) and either
the starting point of propagation (RMIN) if it is set fixed or the
initial wavefunction amplitude in all channels (less than 10**(-IRMSET)).
For the hybrid propagator additional parameters
(RMID or RVFAC, DRAIRY), managing
the transition between the short and the long range propagations, must be
optimized.
We performed the convergence tests of the CC and CS
cross sections of the transition
![]() at 47 cm-1 with the basis set B(5, 0).
All methods converge at better than 0.5% towards a value of
1.435 Å2in CS and 2.265 Å2 in CC; these values are
in slight disagreement with the Phillips et al. (1995) values of 1.48 Å2 in
CS and 2.41 Å2 in CC.
It is difficult to be certain about the origin of this disagreement,
however the convergence
of all our tests to the same values gives us confidence in our results.
It should be noted, when using the hybrid propagator, that a non negative
value of the RVFAC parameter coupled
with an automatic search for the starting point of the short range propagation,
allows an automatic search for the starting point for the long range
propagation.
This association of methods is unstable and requires an optimisation
of the parameter RVFAC at different energies and for each angular
coupling case.
at 47 cm-1 with the basis set B(5, 0).
All methods converge at better than 0.5% towards a value of
1.435 Å2in CS and 2.265 Å2 in CC; these values are
in slight disagreement with the Phillips et al. (1995) values of 1.48 Å2 in
CS and 2.41 Å2 in CC.
It is difficult to be certain about the origin of this disagreement,
however the convergence
of all our tests to the same values gives us confidence in our results.
It should be noted, when using the hybrid propagator, that a non negative
value of the RVFAC parameter coupled
with an automatic search for the starting point of the short range propagation,
allows an automatic search for the starting point for the long range
propagation.
This association of methods is unstable and requires an optimisation
of the parameter RVFAC at different energies and for each angular
coupling case.
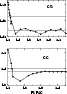 |
Figure 1:
The convergence of the CC and CS
cross sections (in Å2) of the
|
| Open with DEXTER | |
Figure 1 shows the convergence of the
![]() cross section with respect to the parameter
RVFAC. We note that
different optimized values of RVFAC are obtained in CC and CS calculations,
showing that the CC and CS calculations converge for different
values of RVFAC.
Although we were able to obtain convergence, we do not recommend the use of
this
association of methods, i.e. the
use of
cross section with respect to the parameter
RVFAC. We note that
different optimized values of RVFAC are obtained in CC and CS calculations,
showing that the CC and CS calculations converge for different
values of RVFAC.
Although we were able to obtain convergence, we do not recommend the use of
this
association of methods, i.e. the
use of
![]() ,
IRMSET non-zero and RVFAC positive, with the
presently available version of the MOLSCAT code (Hutson & Green 1994).
In the following calculations we choose the
diabatic modified log-derivative method of Manolopoulos (1986)
with or without an automatic search for the short range propagation,
which provides about 1% accuracy in the cross sections and stability
of the parameters from one angular coupling case to the next.
,
IRMSET non-zero and RVFAC positive, with the
presently available version of the MOLSCAT code (Hutson & Green 1994).
In the following calculations we choose the
diabatic modified log-derivative method of Manolopoulos (1986)
with or without an automatic search for the short range propagation,
which provides about 1% accuracy in the cross sections and stability
of the parameters from one angular coupling case to the next.
The state-to-state rotational inelastic rates are the Boltzmann thermal averages of the
state-to-state inelastic cross sections:
We perform CC calculations with a B(j, 2) basis set in the energy range from the opening of the lowest inelastic channel to 381 cm-1 for ortho H2O, and to 380 cm-1 for para H2O. The energies are total energies. We use a B(j, 4) basis set and CS calculations above the energy threshold of the opening of the j2 =2 rotational level of H2. We carefully spanned the energy ranges above the inelastic channels and we added more points in presence of broad resonance structures. For ortho water the energy step is 0.1 cm-1 below 97 cm-1, 0.1, 0.2 or 0.5 cm-1 in an energy range of 20 cm-1 above each inelastic threshold, and small but irregular in the other energy ranges. For para water the energy step is 0.1 cm-1 from 37.2 cm-1 to 75.2 cm-1, 0.2 cm-1 from 75.2 cm-1 to 80 cm-1, 1 cm-1 between 80 cm-1 and 175 cm-1 (with some additional points every 0.2 cm-1 or 0.5 cm-1) and 1 cm-1 or 5 cm-1 above 175 cm-1.
For a collision of H2O with para-H2 Phillips et al. (1995) pointed out that
it is necessary to use a B(j, 2) basis set instead of a B(j, 0) basis set
in the temperature range from 20 K to 140 K.
This is still important at very low temperature;
we find a relative difference of 31% at T=8 K and of 22% at T=20 K
between the collision rates
![]() calculated
respectively with a B(5, 2) and a B(5, 0) basis set.
The j2=2 channel of H2 has a strong influence on the resonance
structure at low energy.
As an example,
Fig. 2 shows the change in the resonance structure of
the CC
calculated
respectively with a B(5, 2) and a B(5, 0) basis set.
The j2=2 channel of H2 has a strong influence on the resonance
structure at low energy.
As an example,
Fig. 2 shows the change in the resonance structure of
the CC
![]() cross section
calculated with and without the j2=2 channel of H2.
cross section
calculated with and without the j2=2 channel of H2.
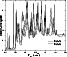 |
Figure 2:
Resonance structures of the CC
|
| Open with DEXTER | |
The presence of overlapping resonances necessitates the use of a very fine
grid of energy points in the thermal Boltzmann average (Eq. (1)) in order
to correctly reproduce the resonance structure.
This is illustrated in Fig. 3 for
the transition
![]() ,
showing that the
collision rate
,
showing that the
collision rate
![]() varies
significantly
and randomly with respect to small changes in the energy
step size. Our best integrals are done by a Simpson rule using integration
energy points which follow the calculated cross sections. Our low-energy step
size of 0.1 cm-1 is particularly small; other published
results (Phillips et al. 1996) at 20 K are usually obtained with sparser energy
grids because of the computing time required.
varies
significantly
and randomly with respect to small changes in the energy
step size. Our best integrals are done by a Simpson rule using integration
energy points which follow the calculated cross sections. Our low-energy step
size of 0.1 cm-1 is particularly small; other published
results (Phillips et al. 1996) at 20 K are usually obtained with sparser energy
grids because of the computing time required.
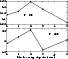 |
Figure 3:
Inelastic rate constants (in cm3 s-1)
of the
|
| Open with DEXTER | |
Another issue is the use of CS calculations in the highest energy range. The CS calculations are relatively accurate for the lowest rotational transitions, but are particularly inaccurate for the largest rotational transitions. Nevertheless the ratio between CC calculations and CS calculations is roughly constant at energies between 328 cm-1 and 381 cm-1, and we use this ratio to scale our CS calculations above 388 cm-1.
Finally we investigate the addition of the j2=4 channel for calculations at energies higher that the opening of the j2=2 channel. The overall effect is negligible at the temperatures considered here.
Tables 3 and 4 give the effective rotational inelastic
rates for ortho and para H2O respectively,
together with the values
obtained by Phillips et al. (1996) at 20 K. These effective rotational
inelastic rates correspond to the sum of the inelastic rates (Eq. (1))
over the final
j2' states for a given initial j2:
We believe that the discrepancies between our rates and the Phillips et al. (1996) rates at 20 K are mainly due to the sparser energy grid used by those authors and that our rate coefficients have an overall accuracy of 3% minimum for all given transitions and temperatures.
For astrophysical use, all our effective excitation rates may be fitted by
the analytical form used by Balakrishnan et al. (1999):
This fitted functions are very different
from the function sometimes used by astrophysicists, when no data are
available:
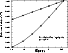 |
Figure 4:
Comparison between the effective calculated CC rates (Eq. (2))
and the rates obtained with Eq. (4),
for the transition
|
| Open with DEXTER | |
Moreover the deexcitation rate of
the
![]() transition increases when the temperature
decreases,
and the deexcitation rates from the 30,3 level increase and
then decrease again when the temperature decreases.
It is therefore not justified to use this simple approximation at very low
temperature.
transition increases when the temperature
decreases,
and the deexcitation rates from the 30,3 level increase and
then decrease again when the temperature decreases.
It is therefore not justified to use this simple approximation at very low
temperature.
| Initial | Final | Points | 5 | 8 | 12 | 16 | 20 | 20 |
| 10,1 | 11,0 | 1033 | 3.92(-14) | 4.34(-13) | 1.71(-12) | 3.36(-12) | 4.99(-12) | 3.06(-12) |
| 10,1 | 21,2 | 680 | 3.69(-18) | 1.54(-15) | 4.41(-14) | 2.37(-13) | 6.52(-13) | 6.10(-13) |
| 10,1 | 22,1 | 441 | 7.74(-26) | 1.35(-20) | 1.12(-17) | 3.25(-16) | 2.46(-15) | 2.42(-15) |
| 10,1 | 30,3 | 424 | 1.42(-25) | 2.89(-20) | 2.60(-17) | 7.82(-16) | 6.04(-15) | 5.88(-15) |
| 10,1 | 31,2 | 353 | 1.86(-31) | 2.03(-24) | 1.70(-20) | 1.56(-18) | 2.34(-17) | 2.26(-17) |
| 10,1 | 32,1 | 272 | 4.73(-36) | 3.30(-27) | 2.74(-22) | 7.95(-20) | 2.40(-18) | 1.74(-18) |
| 10,1 | 33,0 | 92 | 4.03(-46) | 7.54(-34) | 4.95(-27) | 1.26(-23) | 1.40(-21) | 8.57(-22) |
| 10,1 | 41,4 | 211 | 8.56(-37) | 2.23(-27) | 3.81(-22) | 1.57(-19) | 5.78(-18) | 5.98(-18) |
| 10,1 | 42,3 | 62 | 1.00(-47) | 9.20(-35) | 1.47(-27) | 5.91(-24) | 8.61(-22) | 8.05(-22) |
| 11,0 | 10,1 | 1033 | 8.22(-12) | 1.23(-11) | 1.58(-11) | 1.78(-11) | 1.90(-11) | 1.17(-11) |
| 11,0 | 21,2 | 680 | 6.10(-16) | 3.56(-14) | 3.44(-13) | 1.07(-12) | 2.10(-12) | 1.94(-12) |
| 11,0 | 22,1 | 441 | 9.16(-23) | 2.05(-18) | 5.36(-16) | 8.60(-15) | 4.53(-14) | 4.44(-14) |
| 11,0 | 30,3 | 424 | 1.37(-23) | 3.72(-19) | 1.07(-16) | 1.80(-15) | 9.73(-15) | 9.90(-15) |
| 11,0 | 31,2 | 353 | 4.17(-28) | 6.06(-22) | 1.64(-18) | 8.56(-17) | 9.23(-16) | 8.63(-16) |
| 11,0 | 32,1 | 272 | 3.32(-34) | 3.10(-26) | 8.42(-22) | 1.40(-19) | 3.06(-18) | 2.56(-18) |
| 11,0 | 33,0 | 92 | 4.30(-43) | 1.05(-31) | 2.17(-25) | 3.10(-22) | 2.42(-20) | 2.45(-20) |
| 11,0 | 41,4 | 211 | 1.74(-35) | 6.07(-27) | 3.40(-22) | 8.07(-20) | 2.16(-18) | 2.12(-18) |
| 11,0 | 42,3 | 62 | 1.93(-44) | 2.37(-32) | 1.25(-25) | 2.87(-22) | 3.00(-20) | 2.57(-20) |
| 21,2 | 10,1 | 680 | 2.03(-11) | 2.07(-11) | 2.10(-11) | 2.13(-11) | 2.15(-11) | 2.02(-11) |
| 21,2 | 11,0 | 680 | 1.60(-11) | 1.69(-11) | 1.77(-11) | 1.81(-11) | 1.82(-11) | 1.68(-11) |
| 21,2 | 22,1 | 441 | 1.22(-18) | 4.71(-16) | 1.30(-14) | 6.81(-14) | 1.84(-13) | 1.64(-13) |
| 21,2 | 30,3 | 424 | 1.95(-18) | 9.56(-16) | 2.97(-14) | 1.64(-13) | 4.51(-13) | 5.01(-13) |
| 21,2 | 31,2 | 353 | 4.45(-23) | 1.22(-18) | 3.60(-16) | 6.20(-15) | 3.41(-14) | 3.12(-14) |
| 21,2 | 32,1 | 272 | 3.08(-28) | 5.19(-22) | 1.50(-18) | 8.04(-17) | 8.77(-16) | 8.95(-16) |
| 21,2 | 33,0 | 92 | 1.08(-38) | 5.00(-29) | 1.17(-23) | 5.67(-21) | 2.32(-19) | 2.08(-19) |
| 21,2 | 41,4 | 211 | 4.22(-30) | 2.79(-23) | 1.74(-19) | 1.38(-17) | 1.92(-16) | 1.42(-16) |
| 21,2 | 42,3 | 62 | 2.82(-40) | 6.42(-30) | 3.64(-24) | 2.73(-21) | 1.44(-19) | 1.20(-19) |
| 22,1 | 10,1 | 441 | 3.56(-12) | 3.87(-12) | 4.11(-12) | 4.26(-12) | 4.37(-12) | 4.32(-12) |
| 22,1 | 11,0 | 441 | 2.01(-11) | 2.08(-11) | 2.11(-11) | 2.12(-11) | 2.11(-11) | 2.08(-11) |
| 22,1 | 21,2 | 441 | 1.03(-11) | 1.00(-11) | 9.97(-12) | 9.93(-12) | 9.89(-12) | 8.84(-12) |
| 22,1 | 30,3 | 424 | 1.50(-12) | 2.13(-12) | 2.66(-12) | 2.95(-12) | 3.08(-12) | 2.76(-12) |
| 22,1 | 31,2 | 353 | 1.62(-16) | 1.31(-14) | 1.53(-13) | 5.12(-13) | 1.04(-12) | 8.43(-13) |
| 22,1 | 32,1 | 272 | 6.50(-21) | 2.96(-17) | 3.23(-15) | 3.37(-14) | 1.38(-13) | 1.06(-13) |
| 22,1 | 33,0 | 92 | 4.41(-30) | 5.11(-23) | 4.28(-19) | 3.89(-17) | 5.83(-16) | 5.77(-16) |
| 22,1 | 41,4 | 211 | 1.15(-23) | 1.89(-19) | 4.20(-17) | 6.31(-16) | 3.23(-15) | 3.84(-15) |
| 22,1 | 42,3 | 62 | 4.72(-33) | 2.83(-25) | 6.08(-21) | 9.03(-19) | 1.84(-17) | 1.16(-17) |
| 30,3 | 10,1 | 424 | 8.02(-12) | 8.30(-12) | 8.54(-12) | 8.67(-12) | 8.76(-12) | 8.56(-12) |
| 30,3 | 11,0 | 424 | 3.70(-12) | 3.77(-12) | 3.79(-12) | 3.75(-12) | 3.71(-12) | 3.79(-12) |
| 30,3 | 21,2 | 424 | 2.01(-11) | 2.04(-11) | 2.04(-11) | 2.02(-11) | 1.99(-11) | 2.21(-12) |
| 30,3 | 22,1 | 424 | 1.84(-12) | 2.14(-12) | 2.38(-12) | 2.49(-12) | 2.52(-12) | 2.26(-12) |
| 30,3 | 31,2 | 353 | 3.12(-16) | 1.71(-14) | 1.60(-13) | 4.88(-13) | 9.54(-13) | 1.02(-12) |
| 30,3 | 32,1 | 272 | 1.08(-21) | 4.11(-18) | 3.97(-16) | 3.85(-15) | 1.49(-14) | 1.10(-14) |
| 30,3 | 33,0 | 92 | 2.50(-31) | 2.34(-24) | 1.72(-20) | 1.46(-18) | 2.08(-17) | 1.87(-17) |
| 30,3 | 41,4 | 211 | 4.18(-22) | 5.63(-18) | 1.11(-15) | 1.54(-14) | 7.41(-14) | 6.94(-14) |
| 30,3 | 42,3 | 62 | 9.24(-33) | 4.60(-25) | 8.88(-21) | 1.25(-18) | 2.46(-17) | 1.89(-17) |
| Initial | Final | Points | 5 | 8 | 12 | 16 | 20 | 20 |
| 00,0 | 11,1 | 642 | 8.47(-16) | 5.37(-14) | 5.26(-13) | 1.62(-12) | 3.17(-12) | 3.17(-12) |
| 00,0 | 20,2 | 313 | 1.24(-19) | 2.51(-16) | 1.74(-14) | 1.45(-13) | 5.16(-13) | 4.72(-13) |
| 00,0 | 21,1 | 208 | 3.30(-25) | 1.96(-20) | 9.95(-18) | 2.24(-16) | 1.42(-15) | 8.42(-16) |
| 00,0 | 22,0 | 141 | 5.96(-29) | 1.56(-22) | 5.72(-19) | 3.47(-17) | 4.09(-16) | 2.99(-16) |
| 00,0 | 31,3 | 123 | 3.14(-29) | 1.47(-22) | 7.47(-19) | 5.28(-17) | 6.75(-16) | 6.97(-16) |
| 00,0 | 32,2 | 83 | 1.89(-40) | 9.58(-31) | 2.46(-25) | 1.29(-22) | 5.76(-21) | 4.78(-21) |
| 00,0 | 40,4 | 72 | 2.94(-40) | 7.27(-30) | 4.33(-24) | 3.35(-21) | 1.82(-19) | 1.54(-19) |
| 00,0 | 41,3 | 54 | 1.08(-48) | 1.73(-35) | 3.92(-28) | 1.82(-24) | 2.79(-22) | 9.74(-34) |
| 00,0 | 33,1 | 44 | 4.90(-49) | 1.38(-35) | 4.03(-28) | 2.15(-24) | 3.66(-22) | 2.71(-22) |
| 11,1 | 00,0 | 642 | 1.24(-11) | 1.42(-11) | 1.51(-11) | 1.53(-11) | 1.53(-11) | 1.53(-11) |
| 11,1 | 20,2 | 313 | 2.71(-15) | 8.35(-14) | 5.54(-13) | 1.41(-12) | 2.45(-12) | 1.55(-12) |
| 11,1 | 21,1 | 208 | 4.29(-18) | 2.42(-15) | 8.35(-14) | 4.92(-13) | 1.42(-12) | 1.33(-12) |
| 11,1 | 22,0 | 141 | 1.16(-23) | 5.19(-19) | 1.97(-16) | 3.80(-15) | 2.23(-14) | 2.43(-14) |
| 11,1 | 31,3 | 123 | 8.74(-25) | 7.88(-20) | 4.55(-17) | 1.10(-15) | 7.47(-15) | 7.01(-15) |
| 11,1 | 32,2 | 83 | 2.71(-33) | 2.46(-25) | 6.57(-21) | 1.07(-18) | 2.26(-17) | 2.12(-17) |
| 11,1 | 40,4 | 72 | 6.91(-35) | 3.18(-26) | 2.07(-21) | 5.28(-19) | 1.46(-17) | 1.28(-17) |
| 11,1 | 41,3 | 54 | 1.26(-43) | 2.57(-32) | 5.29(-26) | 7.64(-23) | 5.99(-21) | 2.07(-21) |
| 11,1 | 33,1 | 44 | 7.33(-44) | 3.36(-32) | 1.01(-25) | 1.74(-22) | 1.51(-20) | 1.30(-20) |
| 20,2 | 00,0 | 313 | 1.43(-11) | 1.50(-11) | 1.56(-11) | 1.58(-11) | 1.60(-11) | 1.46(-11) |
| 20,2 | 11,1 | 313 | 2.16(-11) | 1.89(-11) | 1.73(-11) | 1.64(-11) | 1.58(-11) | 9.95(-12) |
| 20,2 | 21,1 | 208 | 7.44(-15) | 1.20(-13) | 5.84(-13) | 1.32(-12) | 2.18(-12) | 1.81(-12) |
| 20,2 | 22,0 | 141 | 2.56(-20) | 3.49(-17) | 1.90(-15) | 1.39(-14) | 4.55(-14) | 4.03(-14) |
| 20,2 | 31,3 | 123 | 3.19(-20) | 7.86(-17) | 6.01(-15) | 5.22(-14) | 1.90(-13) | 2.41(-13) |
| 20,2 | 32,2 | 83 | 3.67(-29) | 1.00(-22) | 3.87(-19) | 2.42(-17) | 2.91(-16) | 2.25(-16) |
| 20,2 | 40,4 | 72 | 6.12(-31) | 8.34(-24) | 7.82(-20) | 7.64(-18) | 1.20(-16) | 7.72(-17) |
| 20,2 | 41,3 | 54 | 1.45(-38) | 6.49(-29) | 1.51(-23) | 7.24(-21) | 2.93(-19) | 2.52(-19) |
| 20,2 | 33,1 | 44 | 4.04(-40) | 5.37(-30) | 2.28(-24) | 1.49(-21) | 7.23(-20) | 5.97(-20) |
| 21,1 | 00,0 | 208 | 5.16(-14) | 1.06(-13) | 1.80(-13) | 2.33(-13) | 2.67(-13) | 1.59(-13) |
| 21,1 | 11,1 | 208 | 4.70(-11) | 5.01(-11) | 5.30(-11) | 5.47(-11) | 5.56(-11) | 5.20(-11) |
| 21,1 | 20,2 | 208 | 1.04(-11) | 1.10(-11) | 1.19(-11) | 1.27(-11) | 1.33(-11) | 1.10(-11) |
| 21,1 | 22,0 | 141 | 9.83(-17) | 8.69(-15) | 1.05(-13) | 3.66(-13) | 7.75(-13) | 7.99(-13) |
| 21,1 | 31,3 | 123 | 6.77(-18) | 1.13(-15) | 1.97(-14) | 8.27(-14) | 1.96(-13) | 2.20(-13) |
| 21,1 | 32,2 | 83 | 2.70(-25) | 4.67(-20) | 3.83(-17) | 1.09(-15) | 8.16(-15) | 8.64(-15) |
| 21,1 | 40,4 | 72 | 6.48(-28) | 5.72(-22) | 1.15(-18) | 5.13(-17) | 5.00(-16) | 4.79(-16) |
| 21,1 | 41,3 | 54 | 1.01(-34) | 3.07(-26) | 1.61(-21) | 3.69(-19) | 9.68(-18) | 8.84(-18) |
| 21,1 | 33,1 | 44 | 1.04(-36) | 9.22(-28) | 8.64(-23) | 2.65(-20) | 8.20(-19) | 7.01(-19) |
| 22,0 | 00,0 | 141 | 1.24(-12) | 1.35(-12) | 1.41(-12) | 1.44(-12) | 1.47(-12) | 1.08(-12) |
| 22,0 | 11,1 | 141 | 1.66(-11) | 1.70(-11) | 1.70(-11) | 1.68(-11) | 1.67(-11) | 1.82(-11) |
| 22,0 | 20,2 | 141 | 4.64(-12) | 5.07(-12) | 5.26(-12) | 5.29(-12) | 5.28(-12) | 4.68(-12) |
| 22,0 | 21,1 | 141 | 1.31(-11) | 1.39(-11) | 1.44(-11) | 1.46(-11) | 1.48(-11) | 1.53(-11) |
| 22,0 | 31,3 | 123 | 6.15(-13) | 1.47(-12) | 2.31(-12) | 2.84(-12) | 3.19(-12) | 2.97(-12) |
| 22,0 | 32,2 | 83 | 4.20(-20) | 9.11(-17) | 6.64(-15) | 5.69(-14) | 2.06(-13) | 1.69(-13) |
| 22,0 | 40,4 | 72 | 9.07(-24) | 1.10(-19) | 2.19(-17) | 3.17(-16) | 1.60(-15) | 1.14(-15) |
| 22,0 | 41,3 | 54 | 1.67(-29) | 5.81(-23) | 2.49(-19) | 1.61(-17) | 1.95(-16) | 2.03(-16) |
| 22,0 | 33,1 | 44 | 6.15(-30) | 6.46(-23) | 5.10(-19) | 4.50(-17) | 6.59(-16) | 5.69(-16) |
| 10,1 |
|
|
|
|
|
|
|
|
|
| 0 | -10.3789 | -10.3869 | -10.4242 | -10.1245 | -13.3466 | -10.5093 | -12.0445 | -10.8861 | -11.5006 |
| 1 | -4.7441 | -0.2613 | -5.6338 | -4.8861 | 11.3629 | -9.7223 | -5.8542 | -1.3445 | -7.5809 |
| 2 | 24.9341 | 0.6404 | 17.8475 | 16.4054 | -32.8114 | 29.5880 | 19.9022 | 6.4072 | 23.4461 |
| 3 | -63.5843 | -36.1877 | -96.0049 | -95.6008 | -54.0267 | -158.7750 | -192.9309 | -137.2240 | -205.2970 |
| 4 | 33.6533 | 1.0748 | 14.5757 | 14.0471 | -17.1286 | 21.4216 | 15.8422 | 7.3395 | 16.8892 |
| 11,0 |
|
|
|
|
|
|
|
|
|
| 0 | -10.3790 | -11.3329 | -10.4073 | -11.0068 | -10.3319 | -9.8782 | -11.0402 | -10.6540 | -10.9188 |
| 1 | -4.7429 | 6.3667 | -1.2021 | -1.8128 | -4.7552 | -18.2030 | -9.2674 | -10.5835 | -4.3408 |
| 2 | 24.9290 | -17.4588 | 6.3569 | 9.5160 | 14.9783 | 53.5639 | 31.1035 | 31.8810 | 13.3318 |
| 3 | -51.9665 | -3.4010 | -69.8925 | -76.5337 | -104.3242 | -176.9589 | -196.1172 | -156.7395 | -180.0520 |
| 4 | 33.6462 | -8.1578 | 7.4937 | 10.7398 | 12.6088 | 35.2050 | 22.9118 | 21.6240 | 10.1984 |
| 21,2 |
|
|
|
|
|
|
|
|
|
| 0 | -10.6089 | -11.5549 | -11.3716 | -10.8916 | -10.7837 | -10.5286 | -11.2588 | -10.1292 | -11.5120 |
| 1 | -0.2603 | 6.3682 | 2.5079 | 0.9378 | 1.6764 | -3.5781 | -8.1435 | -8.3748 | -4.2104 |
| 2 | 0.6373 | -17.4630 | -5.9200 | 2.5525 | -2.5947 | 12.5517 | 26.4021 | 25.7030 | 15.0179 |
| 3 | -1.3776 | 19.8017 | -29.3305 | -45.8541 | -60.1146 | -102.3052 | -166.5589 | -126.8035 | -160.9790 |
| 4 | 1.0703 | -8.1609 | -1.0016 | 7.9531 | 3.0985 | 10.9290 | 19.9855 | 19.0032 | 12.6855 |
| 22,1 |
|
|
|
|
|
|
|
|
|
| 0 | -10.6461 | -10.6291 | -11.3724 | -13.0673 | -12.0170 | -10.0915 | -10.0619 | -10.3800 | -10.2047 |
| 1 | -5.6341 | -1.2025 | 2.5147 | 11.2569 | 6.9286 | -3.1601 | -4.4991 | -10.0755 | -10.0538 |
| 2 | 17.8482 | 6.3577 | -5.9381 | -25.8984 | -8.6326 | 11.5113 | 15.6783 | 29.3176 | 28.7513 |
| 3 | -26.5755 | -12.0689 | 5.3138 | 20.0093 | -31.3570 | -67.5097 | -117.5229 | -94.5043 | -141.7895 |
| 4 | 14.5678 | 7.4829 | -1.0202 | -4.1370 | 11.9464 | 11.2662 | 12.6528 | 18.8642 | 19.5192 |
| 30,3 |
|
|
|
|
|
|
|
|
|
| 0 | -10.4976 | -11.3806 | -11.0451 | -13.2231 | -10.4944 | -11.3084 | -11.8466 | -10.5371 | -10.5972 |
| 1 | -4.8374 | -1.7583 | 1.0069 | 11.3465 | -3.4844 | -3.3325 | -2.8068 | 0.1307 | -6.1213 |
| 2 | 16.2259 | 9.3249 | 2.3076 | -26.2015 | 12.5320 | 16.7656 | 11.6927 | 3.0931 | 16.3962 |
| 3 | -24.7090 | -17.2451 | -9.6763 | 21.6127 | -43.2403 | -78.3356 | -111.5536 | -63.4955 | -123.5756 |
| 4 | 13.8780 | 10.5731 | 7.7369 | -4.3454 | 11.8678 | 19.0277 | 10.2459 | 6.1810 | 10.6898 |
| 00,0 |
|
|
|
|
|
|
|
|
|
| 0 | -9.9974 | -10.4731 | -15.8034 | -9.6994 | -10.9421 | -6.4746 | -11.2512 | -14.6925 | -13.3543 |
| 1 | -4.1238 | 2.6873 | 29.8017 | -11.6981 | 0.6573 | -57.8975 | -4.5555 | 10.2500 | 5.5504 |
| 2 | 16.6079 | -6.0654 | -72.3553 | 36.0829 | 1.3266 | 172.7658 | 13.8900 | -11.8564 | -12.2570 |
| 3 | -49.8872 | -39.5805 | 0.5622 | -134.7151 | -94.7562 | -360.7653 | -157.5076 | -188.2216 | -167.9145 |
| 4 | 13.9732 | -0.1119 | -12.5992 | 24.9535 | 4.7175 | 116.9532 | 9.7020 | 20.7752 | -3.6496 |
| 11,1 |
|
|
|
|
|
|
|
|
|
| 0 | -10.4760 | -11.3828 | -10.5967 | -10.1736 | -10.3632 | -11.4142 | -11.2061 | -13.4431 | -11.9383 |
| 1 | -4.1109 | 5.1435 | 4.5018 | -4.2417 | -3.5859 | -0.4472 | 0.7173 | 4.7008 | -1.5315 |
| 2 | 16.5666 | -12.8492 | -11.8574 | 15.9061 | 11.2659 | 5.0774 | -0.5198 | -6.3091 | 6.5207 |
| 3 | -26.6238 | -5.2013 | -24.7220 | -86.3470 | -83.1425 | -118.5232 | -117.2276 | -157.9642 | -166.4029 |
| 4 | 13.9434 | -6.4753 | -3.4580 | 13.2265 | 10.0649 | 9.2072 | 2.1318 | 13.1971 | 6.4090 |
| 20,2 |
|
|
|
|
|
|
|
|
|
| 0 | -11.1739 | -11.6110 | -10.9854 | -10.9788 | -10.5715 | -10.7710 | -10.4586 | -12.7111 | -13.1334 |
| 1 | 2.7033 | 5.2053 | 3.0807 | -3.7934 | -0.2219 | -3.9628 | -5.3168 | 4.5269 | 5.8649 |
| 2 | -6.1182 | -13.0745 | -13.6358 | 15.7831 | 4.0774 | 13.6220 | 15.7231 | -11.8668 | -16.8304 |
| 3 | 4.2901 | 15.7584 | 4.5238 | -67.6138 | -54.9645 | -108.2711 | -117.2091 | -115.8773 | -114.2936 |
| 4 | -0.1446 | -6.6583 | -10.1398 | 14.7956 | 6.8654 | 14.0995 | 12.1368 | -4.6166 | -9.1990 |
| 21,1 |
|
|
|
|
|
|
|
|
|
| 0 | -16.4968 | -10.8369 | -10.9983 | -9.8342 | -10.3130 | -10.7596 | -11.3061 | -11.0861 | -12.0892 |
| 1 | 29.7469 | 4.6805 | 3.2181 | -8.6413 | -7.1411 | 0.5537 | -0.7842 | -2.1446 | -0.5204 |
| 2 | -72.1527 | -12.5063 | -14.1846 | 28.4892 | 21.2772 | 1.2362 | 3.9445 | 6.9918 | 2.5470 |
| 3 | 59.6970 | 12.5799 | 21.1504 | -67.4964 | -58.5062 | -76.1090 | -86.4823 | -124.3064 | -124.2489 |
| 4 | -12.3982 | -3.9974 | -10.6578 | 22.5416 | 14.9127 | 5.7028 | 4.4782 | 6.9657 | 3.3339 |
| 22,0 |
|
|
|
|
|
|
|
|
|
| 0 | -10.3972 | -10.3940 | -10.9777 | -9.8348 | -10.4334 | -10.5110 | -12.6026 | -11.8090 | -10.6685 |
| 1 | -11.7096 | -4.2531 | -3.8024 | -8.6350 | -9.1989 | -0.0096 | 7.1506 | 2.2873 | 0.4973 |
| 2 | 36.1275 | 15.9426 | 15.8180 | 28.4687 | 34.6227 | 2.3748 | -27.5952 | -2.6543 | 0.8718 |
| 3 | -49.6940 | -24.5160 | -26.3855 | -41.8535 | -58.1543 | -52.4147 | -14.3669 | -88.8274 | -97.3576 |
| 4 | 24.9949 | 13.2622 | 14.8394 | 22.5386 | 29.0011 | 6.9352 | -19.7570 | 3.1452 | 2.9082 |
This paper is a continuation of the work of Phillips and co-workers
(Green et al. 1993; Phillips et al. 1994, 1995, 1996) on the excitation
rates of H2O by H2.
Phillips et al. (1996) gave excitation rates in the energy
range from 20 K to 140 K; we calculated these rates in the energy range
from 5 K to 20 K. We also provided fits of the excitation rates
that are valid in the energy range from 5 K to 20 K. The results
presented in this paper, the excitation rates of other transitions,
and the associated cross sections will be made available on our
WEB site![]() .
.
We carried out a thorough investigation of the energy dependence of the excitation cross sections, which showed the importance of the resonance structure at low temperature. The resonances are certainly both Feshbach and shape resonances, and the resonance structure is very strongly linked to the potential energy surface. An assessment of the accuracy of the PES and an improvement of the potential energy surface including the vibrational degrees of freedom are underway (P. Valiron et al., in preparation).
Acknowledgements
The authors thank C. Ceccarelli for pointing out the interest of these calculations and E. Roueff and P. Valiron for stimulating discussions. Most scattering calculations were performed at the IDRIS-CNRS (Institut du developpement et des ressources en informatique scientifique du Centre National de la Recherche Scientifique) under project 011472-CP:4.