We present a CSO and BIMA molecular line survey of the dense, quiescent molecular environment ahead of HH 2. The molecular gas is cold, 13 K, and moderately dense, 3
A&A 388, 1004-1015 (2002)
DOI: 10.1051/0004-6361:20020536
J. M. Girart1 - S. Viti2 - D. A. Williams2 - R. Estalella1 - P. T. P. Ho3
1 -
Departament d'Astronomia i Meteorologia, Universitat de Barcelona,
Av. Diagonal 647, 08028 Barcelona, Catalunya, Spain
2 -
Department of Physics and Astronomy, University College London,
London, WC1E 6BT, England, UK
3 -
Harvard-Smithsonian Center for Astrophysics, 60 Garden Street, Cambridge,
MA 02138, USA
Received 27 December 2001 / Accepted 4 April 2002
Abstract
We present a CSO and BIMA molecular line survey of the dense, quiescent
molecular environment ahead of HH 2. The molecular gas is cold, 13 K, and
moderately dense, 3
![]() cm-3. A total of 14 species has been detected
(including different isotopes and deuterated species). The relative abundances
of the clump are compared with other dense molecular environments, including
quiescent dark clouds, and active low and high mass star forming regions. This
comparison confirms the peculiar chemical composition of the quiescent gas
irradiated by the HH objects. Thus, from this comparison, we found that the
HCO+, CH3OH and H2CO are strongly enhanced.
SO and SO2 are weakly enhanced, whereas HCN and CS are underabundant.
The CN abundance is within the range of values found in starless dark clouds,
but it is low with respect to high mass star forming regions.
Finally, the chemical composition of
HH 2 confirms the qualitative results of the Viti & Williams (1999) complex
chemical model that follows the chemical behavior of a molecular clump
irradiated by a HH object.
cm-3. A total of 14 species has been detected
(including different isotopes and deuterated species). The relative abundances
of the clump are compared with other dense molecular environments, including
quiescent dark clouds, and active low and high mass star forming regions. This
comparison confirms the peculiar chemical composition of the quiescent gas
irradiated by the HH objects. Thus, from this comparison, we found that the
HCO+, CH3OH and H2CO are strongly enhanced.
SO and SO2 are weakly enhanced, whereas HCN and CS are underabundant.
The CN abundance is within the range of values found in starless dark clouds,
but it is low with respect to high mass star forming regions.
Finally, the chemical composition of
HH 2 confirms the qualitative results of the Viti & Williams (1999) complex
chemical model that follows the chemical behavior of a molecular clump
irradiated by a HH object.
Key words: ISM: individual objects: HH 2 - ISM: abundances - ISM: clouds - ISM: molecules - radio lines: ISM - stars: formation
Herbig-Haro (HH) objects, which trace shock-excited plasma, are signposts of
the intense outflow phenomena associated with star formation (e.g.,
Reipurth & Bally 2001). Several HH objects are found to have
associated quiescent dense clumps ahead of them: HH 1/2 (see references below),
HH 7-11 (Rudolph & Welch 1988; Dent et al. 1993; Rudolph
et al. 2001), HH 34 (Rudolph & Welch 1992), NGC 2264G
(Girart et al. 2000), HH 80N (Girart et al. 1994,
1998, 2001). Most of them have
high excitation knots, i.e., with strong high excitation lines, such as [OIII]
and H![]() ,
strong UV lines and significant UV continuum radiation (e.g.,
Reipurth & Bally 2001). The quiescent clumps are characterized
by the low temperature and narrow line widths of the molecular emission, and
by the emission enhancement of HCO+ and NH3. In spite of the presence of the
HH objects, there is a lack of apparent dynamical perturbation in the clumps.
However, recent observations show clear signposts of star formation within the
HH 80N clumps (Girart et al. 2001).
,
strong UV lines and significant UV continuum radiation (e.g.,
Reipurth & Bally 2001). The quiescent clumps are characterized
by the low temperature and narrow line widths of the molecular emission, and
by the emission enhancement of HCO+ and NH3. In spite of the presence of the
HH objects, there is a lack of apparent dynamical perturbation in the clumps.
However, recent observations show clear signposts of star formation within the
HH 80N clumps (Girart et al. 2001).
Girart et al. (1994) suggested that the regions of enhanced HCO+and NH3 emission were a consequence of the irradiation from the HH shock affecting the chemistry within a small dense clump nearby in the molecular cloud. It was supposed that the UV radiation would evaporate icy mantles and promote photochemistry in the enriched gas. Taylor & Williams (1996) modeled this situation with a simple chemistry and showed that HCO+ would arise from the interaction of C+ (derived from CO) with H2O liberated from the ice. The chemical enhancements would be transient, but predicted column densities were significant. Viti & Williams (1999, hereafter VW99) noted that the impact of a high radiation field on a dense gas should lead to a rich chemistry, and they therefore extended the 1996 model. They predicted that, in addition to HCO+ and NH3, a wide variety of species should arise with enhanced abundances, including CH3OH, H2CO, SO, SO2, and CN and that the effects in a single clump should last for 104 years. Raga & Williams (2000) showed that the photochemical effects created by the HH object should respond to its movement through the molecular cloud, and predicted that the enhanced regions should have a characteristic morphology.
| Beam |
|
|
|
|||
| Molecule | Transition | (GHz) | Size | (K km s-1) | ( km s-1) | ( km s-1) |
| CS | 5-4 | 244.93561 | 31'' | 0.17 |
6.4 |
2.4 |
| C3H2 | 33,0-22,1 | 216.27873 | 34'' | |
|
|
| HCN | 3-2 | 265.88643 | 28'' | 0.65 |
6.43 |
2.6 |
| HCO+ | 3-2 | 267.55763 | 28'' | 7.53 |
6.84 |
1.83 |
| HCO+ | 4-3 | 356.73425 | 21'' | 3.80 |
6.91 |
1.84 |
| H13CO+ | 3-2 | 260.25548 | 29'' | 0.31 |
7.06 |
1.30 |
| HCS+ | 5-4 | 213.36053 | 35'' | |
|
|
| H2CO | 30,3-20,2 | 218.22219 | 34'' | 1.23 |
6.73 |
1.77 |
| H2CO | 50,5-40,4 | 362.73605 | 21'' | |
|
|
| H2CS | 70,7-60,6 | 240.26616 | 31'' | |
|
|
| H2S | 22,0-21,1 | 216.71044 | 34'' | |
|
|
| CH3OH | 50-40 E | 241.70017 | 31'' | |
|
|
| CH3OH | 5-1-4-1 E | 241.76722 | 31'' | 0.21 |
6.54 |
1.24 |
| CH3OH | 50-40 A+ | 241.79143 | 31'' | 0.22 |
6.54 |
1.24 |
| CH3OH | 7-1-6-1 E | 338.34463 | 22'' | |
|
|
| CH3OH | 70-60 A+ | 338.40868 | 22'' | |
|
|
| CH3OH | 71-61 E | 338.61500 | 22'' | |
|
|
| NS |
|
253.57048 | 30'' | |
|
|
| NS |
|
253.57215 | 30'' | |
|
|
| SO | 65-54 | 219.94939 | 34'' | 0.70 |
6.52 |
1.26 |
| SO | 76-65 | 261.84370 | 29'' | 0.31 |
6.62 |
1.16 |
|
a b Overlapped with the NS 11/2,11/2-9/2,9/2 transition. |
Implicit in the model is the idea that molecular clouds are clumpy on a small scale. This was suggested by Taylor et al. (1996) as a necessary assumption to interpret the relative spatial distributions of CS and NH3 in molecular clouds in terms of time-dependent chemistry. The clumps were required to be transient on a timescale of about one million years. Subsequent interferometer observations (e.g. Peng et al. 1998, for TMC-1 Core D; Morata et al. 2002 for L673) have supported the Taylor et al. model. Therefore, the HH objects may be regarded as probes of the transient substructure of molecular clouds. Wherever the probe encounters a small clump, a characteristic chemistry arises. This paper reports a search for that characteristic chemistry in the clump associated with HH 2.
The prototypical HH 1 and HH 2 objects (Herbig 1951; Haro 1952), located in the L1641N molecular cloud in Orion, provided the earliest spectral evidence that the HH objects trace strong shocks due to the presence of stellar winds (Schwartz 1978). Both HH 1 and HH 2 belong to the class of high excitation HH objects, with strong UV (Böhm et al. 1987; Raymond et al. 1988; Raymond et al. 1997) and centimeter continuum emission (Rodríguez et al. 1990, 2000). HH 2 shows a very complex and apparently chaotic morphological, kinematical and excitation structure (e.g., Schwartz et al. 1993; Eislöffel et al. 1994). Detailed HST observations reveal that this complex structure is the consequence of the interaction of the HH 2 jet with a dense ambient cloud, resulting in a very bright, high ionization and complex structure (Hester et al. 1998). Recent Chandra observations reveal that the strongest HH 2 knot, which is also the highest excitation knot, has associated X-ray emission (Pravdo et al. 2001).
The presence of quiescent dense ambient gas ahead of HH 2 has been well
established from HCO+ and NH3 observations (Davis et al. 1990; Torrelles et al. 1992, 1994; Choi & Zhou
1997). The ammonia emission shows a clumpy medium, with clump sizes
of only 20'' (Torrelles et al. 1992) or ![]() 8000 AU (assuming a
distance of 390 pc: Anthony-Twarog 1982). JCMT observations by
Dent (1997) shows that the chemical composition of the dense ambient
gas is strongly altered by the presence of the HH 2 radiation, with a strong
emission enhancement of the HCO+. Wolfire & Königl (1993)
carried out a shock model where the X-ray, EUV and FUV radiation field from
HH 2 penetrates the cloud, initiates an ion chemistry, enhancing the
abundances of the HCO+ and the electrons, which produces an excitation
enhancement of the HCO+. The combination of these two effects lead to a
strong emission enhancement.
8000 AU (assuming a
distance of 390 pc: Anthony-Twarog 1982). JCMT observations by
Dent (1997) shows that the chemical composition of the dense ambient
gas is strongly altered by the presence of the HH 2 radiation, with a strong
emission enhancement of the HCO+. Wolfire & Königl (1993)
carried out a shock model where the X-ray, EUV and FUV radiation field from
HH 2 penetrates the cloud, initiates an ion chemistry, enhancing the
abundances of the HCO+ and the electrons, which produces an excitation
enhancement of the HCO+. The combination of these two effects lead to a
strong emission enhancement.
In this paper we present 0.8-1.4 mm CSO observations towards the quiescent
clump ahead of HH 2. We also present 3 mm spectra obtained with the BIMA
array. The goal of this paper is to study the chemical effect produced by the
strong HH 2 radiation over the dense quiescent ambient gas around HH 2. A
second paper will analyze and discuss the spatial distribution of the
molecules observed with the BIMA array (Girart et al. 2002, hereafter
Paper II).
![\begin{figure}
\par\includegraphics[width=10cm,clip]{MS2228f1.eps} \end{figure}](/articles/aa/full/2002/24/aa2228/Timg20.gif) |
Figure 1: Spectra of the detected lines with the BIMA and CSO telescopes. Lines from BIMA were convolved with a Gaussian in order to obtain a resulting beam of 30'', approximately the beam of the CSO data at 250 GHz. |
| Open with DEXTER | |
|
|
|
|
|||
| Molecule | Transition | (GHz) | (K km s-1) | ( km s-1) | ( km s-1) |
| CH3CN | 61-51 F = 7-6 | 110.38140 | |
|
|
| CH3CN | 60-50 F = 7-6 | 110.38352 | |
|
|
| CH3SH | 30-20 A+ | 75.86293 | |
|
|
| CH3OH | 2-1-1-1 E | 96.73939 | 0.33 |
6.57 |
0.86 |
| CH3OH | 20-10 A+ | 96.74143 | 0.47 |
id. | id. |
| CH3OH | 20-10 E | 96.74458 | 0.06 |
id. | id. |
| CH3OH | 21-11 E | 96.75551 | id. | id. | |
| CN | 1-0
|
113.19132 | 0.09 |
7.10 |
1.2 |
| CN | 1-0
|
113.48814 | |
|
|
| CN | 1-0
|
113.49098 | 0.17 |
6.77 |
1.5 |
| CN | 1-0
|
113.49964 | 0.12 |
id. | id. |
| C18O | 1-0 | 109.78216 | 0.38 |
6.43 |
0.53 |
| 13CO | 1-0 | 110.20135 | 4.38 |
7.09 |
1.76 |
| CS | 2-1 | 97.98097 | 0.55 |
6.51 |
1.72 |
| C3H2 | 21,2-10,1 | 85.33890 | |
|
|
| DCN | 1-0 F = 2-1 | 72.41491 | 0.51 |
6.78 |
1.17 |
| DCO+ | 1-0 | 72.03933 | 0.74 |
6.59 |
0.76 |
| HC18O+ | 1-0 | 85.16226 | 0.07 |
6.9 |
1.6 |
| HCN | 1-0 F = 1-1 | 88.63042 | 0.60 |
6.44 |
1.97 |
| HCN | 1-0 F = 2-1 | 88.63185 | 1.40 |
id. | id. |
| HCN | 1-0 F = 0-1 | 88.63394 | 0.36 |
id. | id. |
| HCO+ | 1-0 | 89.18852 | 8.48 |
6.83 |
1.73 |
| HCOOH | 40,4-30,3 | 89.57917 | |
|
|
| HCS+ | 2-1 | 85.34790 | |
|
|
| HNCO | 50,5-40,4 | 109.90576 | |
|
|
| H2CO | 10,1-00,0 | 72.83795 | 2.46 |
6.54 |
1.15 |
| H2CO | 51,4-51,5 | 72.40909 | |
|
|
| H2CS | 31,3-21,2 | 101.47775 | |
|
|
| H2CS | 30,3-20,2 | 103.04040 | |
|
|
| H2CS | 32,1-22,0 | 103.05179 | |
|
|
| HC3N | 11-10 | 100.07639 | |
|
|
| NH2CN | 51,4-41,3 | 100.62950 | |
|
|
| OCS | 8-7 | 97.30121 | |
|
|
| SO | 22-11 | 86.09399 | 0.44 |
6.47 |
0.79 |
| SO | 32-21 | 99.29988 | 1.77 |
6.45 |
1.01 |
| SO | 45-44 | 100.02957 | |
|
|
| SO | 23-12 | 109.25218 | 0.35 |
6.60 |
0.89 |
| SO2 | 60,6-51,5 | 72.75824 | 0.26 |
6.8 |
1.3 |
| SO2 | 101,9-92,8 | 76.41217 | |
|
|
| SO2 | 31,3-20,2 | 104.02942 | 0.22 |
6.59 |
0.97 |
| SO2 | 101,9-100,10 | 104.23929 | |
|
|
Observations were carried out in October 1999 with the 10.4 m telescope of the
Caltech Submillimeter Observatory![]() (CSO). The 200-300 GHz and 300-400 GHz receivers were used in
conjunction with 50 MHz and 500 MHz acousto-optical spectrometers, which
provided velocity coverage of 60 and 600 km s-1 at 250 GHz and of 40 and
400 km s-1 at 350 GHz. Pointing, focus and calibration were checked every few
hours using Saturn. The main-beam efficiency, measured by observing Saturn,
were
(CSO). The 200-300 GHz and 300-400 GHz receivers were used in
conjunction with 50 MHz and 500 MHz acousto-optical spectrometers, which
provided velocity coverage of 60 and 600 km s-1 at 250 GHz and of 40 and
400 km s-1 at 350 GHz. Pointing, focus and calibration were checked every few
hours using Saturn. The main-beam efficiency, measured by observing Saturn,
were ![]() 67% at 250 GHz and
67% at 250 GHz and ![]() 60% at 350 GHz and system temperatures
ranged 240-670 K. All the spectra were observed at the position:
60% at 350 GHz and system temperatures
ranged 240-670 K. All the spectra were observed at the position:
![]() ;
;
![]() .
This position is very close (
.
This position is very close (
![]() )
to the HH 2 knot L (e.g., Hester et al. 1998) and to the peak emission of the HCO+ 4-3 integrated
map from Dent (1997).
Table 1 lists all the molecular lines observed along with their rest
frequency, the angular resolution at the observed frequency and the Gaussian
fits to the line profiles. The upper limits show the 3-
)
to the HH 2 knot L (e.g., Hester et al. 1998) and to the peak emission of the HCO+ 4-3 integrated
map from Dent (1997).
Table 1 lists all the molecular lines observed along with their rest
frequency, the angular resolution at the observed frequency and the Gaussian
fits to the line profiles. The upper limits show the 3-![]() level within
the 5.2-7.8 km s-1 range.
level within
the 5.2-7.8 km s-1 range.
The 10-antenna BIMA array![]() observations were carried out between 1999 October and
2001 May in the C configuration. A detailed description of the observations
and reduction is given in Paper II. In order to compare with the CSO spectra,
we smoothed the BIMA maps by convolving them with a Gaussian, with a resulting
FWHM beam of 30'', the angular resolution of the CSO observations at
230 GHz. A spectrum of each transition observed with BIMA was synthesized at
the position observed with the CSO telescope (see paragraph above).
Table 2 lists all the molecular lines observed, their rest
frequency, and the Gaussian fits to the line profiles.
observations were carried out between 1999 October and
2001 May in the C configuration. A detailed description of the observations
and reduction is given in Paper II. In order to compare with the CSO spectra,
we smoothed the BIMA maps by convolving them with a Gaussian, with a resulting
FWHM beam of 30'', the angular resolution of the CSO observations at
230 GHz. A spectrum of each transition observed with BIMA was synthesized at
the position observed with the CSO telescope (see paragraph above).
Table 2 lists all the molecular lines observed, their rest
frequency, and the Gaussian fits to the line profiles.
Figure 1 shows the spectra of the detected lines with the CSO and
the BIMA observations smoothed to the 30'' angular resolution. A total of 33
transitions (including hyperfine transitions) were detected for 14 species:
13CO and C18O, HCO+, H13CO+, HC18O+, HCN, H2CO, CN, CH3OH, CS, SO, SO2 and
the deuterated species DCO+ and DCN. The spectral lines peak at a
![]() within the range of
within the range of ![]() 6.4 km s-1 (CS and HCN) to
6.4 km s-1 (CS and HCN) to ![]() 6.9 km s-1 (HCO+).
This range of values is in agreement with previous observations of the
quiescent gas ahead of HH 2 (Torrelles et al. 1992; Choi & Zhou
1997). The line widths range from
6.9 km s-1 (HCO+).
This range of values is in agreement with previous observations of the
quiescent gas ahead of HH 2 (Torrelles et al. 1992; Choi & Zhou
1997). The line widths range from ![]() 0.8 km s-1 for the DCO+ to
0.8 km s-1 for the DCO+ to
![]() 1.9 km s-1 for HCO+ and HCN. The 1 mm CS and HCN transitions have large
line widths,
1.9 km s-1 for HCO+ and HCN. The 1 mm CS and HCN transitions have large
line widths, ![]() 2.4 km s-1. However, this value is not significant due to the
low SNR of these lines. The differences in line center velocity and line width
are significant and are due to the combination of velocity gradients within the
quiescent ambient gas and a spatial chemical differentiation, which will be
discussed in Paper II. Yet, for the HCO+, the larger line width can also be due
to a high optical depth and the presence of high velocity emission. The HCO+ 1-0 spectrum shows clearly redshifted and blueshifted wings, which
implies the presence of high velocity molecular gas. This high velocity
component is tracing the interaction between the HH object and the clump,
which confirms the spatial association of the clump with HH 2 (Paper II).
2.4 km s-1. However, this value is not significant due to the
low SNR of these lines. The differences in line center velocity and line width
are significant and are due to the combination of velocity gradients within the
quiescent ambient gas and a spatial chemical differentiation, which will be
discussed in Paper II. Yet, for the HCO+, the larger line width can also be due
to a high optical depth and the presence of high velocity emission. The HCO+ 1-0 spectrum shows clearly redshifted and blueshifted wings, which
implies the presence of high velocity molecular gas. This high velocity
component is tracing the interaction between the HH object and the clump,
which confirms the spatial association of the clump with HH 2 (Paper II).
Figure 2 shows the distribution of the quiescent molecular gas, traced by the 13CO emission, ahead of the HH 2 region. There is clear column density enhancement, mainly downstream of HH2, whereas at the position of HH 2 or just behind it there is little ambient gas. The SO emission is also shown to better locate the chemically enhanced region just ahead of HH 2, almost coincident with HH 2 knot L (e.g., Hester et al. 1998). The high excitation HH 2 emission, as well as the X-ray and the radio continuum emission, arise from the brightest and largest knot in the [SII] image (see Fig. 2).
HCO+, SO and CH3OH are the molecules with more than three transitions detected (including the rarer isotopes for the HCO+). Therefore, the observed emission from these molecules can be used to derive the physical condition of the quiescent gas. SO and CH3OH are molecules with moderate dipole moments, 1.55 and 0.90 Debyes, respectively, which implies that if the densities are not too low their emission will be close to thermalization. Thus, we carried out the population diagram analysis (e.g., Goldsmith & Langer 1999) to derive the temperature of the gas and the column densities of SO and CH3OH. For the HCO+, since we have two rare isotopes observed, H13CO+ and HC18O+, we carried out the Monte Carlo radiative transfer model developed by Hogerheijde & van der Tak (2000).
 |
Figure 2:
Superposition of the gray scale of the [SII] image (from Curiel, private
communication), the integrated emission of the 13CO 1-0 line (thin
contours) over the 5.1-8.1 km s-1
|
| Open with DEXTER | |
The method used to carry out the population diagram analysis, which takes into account approximately the optical depth, is described in Appendix A. In order to obtain more accurate fits for the SO and CH3OH, which were observed with two different telescopes, a 20% calibration uncertainty was included in the estimated errors. Figure 3 shows the population diagram for the SO and CH3OH molecules.
![\begin{figure}
\par\includegraphics[width=8.8cm,clip]{MS2228f3.eps} \end{figure}](/articles/aa/full/2002/24/aa2228/Timg31.gif) |
Figure 3:
Population diagrams for the SO (left panel) and the CH3OH (right panel).
Solid filled circles indicate the observed data. The error bars include the
measurement uncertainty and the calibration uncertainty ( |
| Open with DEXTER | |
The SO emission is best fitted for
![]() K,
K,
![]() cm-2 and a filling factor of
f=0.35. This solution gives moderate optical depths for the observed
transitions, with a maximum value of 1.0 for the 32-21 transition.
The high angular resolution (
cm-2 and a filling factor of
f=0.35. This solution gives moderate optical depths for the observed
transitions, with a maximum value of 1.0 for the 32-21 transition.
The high angular resolution (
![]() )
BIMA SO
32-21 maps show that the integrated emission arises from a region
with a deconvolved size of
)
BIMA SO
32-21 maps show that the integrated emission arises from a region
with a deconvolved size of
![]() ,
,
![]() (Paper II), which
implies a filling factor of approximately
(Paper II), which
implies a filling factor of approximately
![]() .
Therefore, the best solution for the SO
obtained using the method described in Appendix A, gives a
filling factor in agreement with the observed value from the BIMA maps.
.
Therefore, the best solution for the SO
obtained using the method described in Appendix A, gives a
filling factor in agreement with the observed value from the BIMA maps.
The population diagram technique for the CH3OH emission gives the best fit
for
![]() K,
N(CH3OH)
K,
N(CH3OH)
![]() cm-2, and f=0.20. The
optical depths of the observed transitions are small: the maximum value is
0.56, for the 20-10 A+ transition. The smaller filling factor of
the CH3OH solution with respect to the SO is because of its slightly smaller
emitting area:
cm-2, and f=0.20. The
optical depths of the observed transitions are small: the maximum value is
0.56, for the 20-10 A+ transition. The smaller filling factor of
the CH3OH solution with respect to the SO is because of its slightly smaller
emitting area:
![]() (Paper II). We note that although
the optical depths of the SO and CH3OH are small (
(Paper II). We note that although
the optical depths of the SO and CH3OH are small (![]() 1) the population
diagram technique used here and described in Appendix A gives column
densities of 20% (CH3OH) to 30% (SO) higher than the standard population
diagram technique where optically thin emission is assumed.
1) the population
diagram technique used here and described in Appendix A gives column
densities of 20% (CH3OH) to 30% (SO) higher than the standard population
diagram technique where optically thin emission is assumed.
We used the one dimensional version of the Monte Carlo code developed by Hogerheijde & van der Tak (2000), which calculates the radiative transfer and excitation of molecular lines. The code is formulated from the viewpoint of cells rather than photons, which allows the separation of local and external contributions of the radiation field. This gives an accurate and fast performance even for high opacities (for more details see Hogerheijde & van der Tak 2000).
![\begin{figure}
\par\includegraphics[width=9cm,clip]{MS2228f4.eps} \end{figure}](/articles/aa/full/2002/24/aa2228/Timg41.gif) |
Figure 4: Observed (solid contours) and synthetic (dashed contours) HCO+, H13CO+ and HC18O+ spectra. |
| Open with DEXTER | |
The HCO+ emission was assumed to arise from a sphere of radius
![]() cm or 6680 AU (17'' at the distance of HH 2), which is approximately
the radius of the BIMA HCO+ 1-0 emission for the peak intensity
component (the HCO+ emission extends over a larger region to the NE of the
peak intensity, but this extended component is not picked up by the CSO beam).
The volume density and temperature were assumed constant and no velocity
gradient was adopted (we note that the results are insensitive for radial
velocity gradients of
cm or 6680 AU (17'' at the distance of HH 2), which is approximately
the radius of the BIMA HCO+ 1-0 emission for the peak intensity
component (the HCO+ emission extends over a larger region to the NE of the
peak intensity, but this extended component is not picked up by the CSO beam).
The volume density and temperature were assumed constant and no velocity
gradient was adopted (we note that the results are insensitive for radial
velocity gradients of ![]() 0.3 km s-1 within the adopted radius, i.e.,
0.3 km s-1 within the adopted radius, i.e., ![]() 9 km s-1 pc-1). The 12C16O to 13C16O and
12C16O to 12C18O abundance ratios adopted were 63 and 560,
derived for the Orion region by Langer & Penzias (1990,
1993). We explored a large range of values for the volume density,
the temperature, the molecular fractional abundance and the intrinsic line
width. The resulting synthetic maps of the different transitions were convolved
with a Gaussian to match the angular resolution of the spectra (28'' for the
1-0 and 3-2 transitions and 21'' for the 4-3 transition).
The best fit is obtained for a volume density of
9 km s-1 pc-1). The 12C16O to 13C16O and
12C16O to 12C18O abundance ratios adopted were 63 and 560,
derived for the Orion region by Langer & Penzias (1990,
1993). We explored a large range of values for the volume density,
the temperature, the molecular fractional abundance and the intrinsic line
width. The resulting synthetic maps of the different transitions were convolved
with a Gaussian to match the angular resolution of the spectra (28'' for the
1-0 and 3-2 transitions and 21'' for the 4-3 transition).
The best fit is obtained for a volume density of
![]() cm-3, a
temperature of
cm-3, a
temperature of ![]() 13 K, an intrinsic line width of
13 K, an intrinsic line width of ![]() 0.6 km s-1, and a
HCO+ fractional abundance of
0.6 km s-1, and a
HCO+ fractional abundance of
![]() .
Figure 4 shows the
overlap of the observed and synthetic spectra for the HCO+, H13CO+ and HC18O+ transitions. The synthetic spectra fit well the observed spectra, except for
the HCO+ 1-0. The lack of observed emission at the central and
redshifted sections of the line, as compared with the predicted spectra, is
possibly due to a combination of two effects. First, it could be due to the
missing flux, a consequence of the lack of short spacing visibilities at the
interferometer. Second, it is possible that the HCO+ 1-0 is affected
by the absorption of a cold and low density component of the cloud, whose
effects will only be observable in the lowest rotational transition of
abundant species with high dipole moment (see Girart et al. 2000).
.
Figure 4 shows the
overlap of the observed and synthetic spectra for the HCO+, H13CO+ and HC18O+ transitions. The synthetic spectra fit well the observed spectra, except for
the HCO+ 1-0. The lack of observed emission at the central and
redshifted sections of the line, as compared with the predicted spectra, is
possibly due to a combination of two effects. First, it could be due to the
missing flux, a consequence of the lack of short spacing visibilities at the
interferometer. Second, it is possible that the HCO+ 1-0 is affected
by the absorption of a cold and low density component of the cloud, whose
effects will only be observable in the lowest rotational transition of
abundant species with high dipole moment (see Girart et al. 2000).
The assumed radius, derived from the HCO+ 1-0 maps, could be highly uncertain due to high optical depth, absorption by the foreground cold gas or resolving out the large-scale HCO+ emission. However, the models run with radii higher or lower than roughly 30% of the value assumed do not fit the observed spectra. For small changes in the radii the best solutions are always in the 12-14 K temperature range.
The temperature derived here is only slightly higher than the temperature
derived from the SO and CH3OH population diagrams. This implies that the
observed SO and CH3OH transitions are close to thermalization for the derived
volume density,
![]() cm-3.
cm-3.
From the previous analyses the derived temperature of the cloud is ![]() 13 K.
Yet, the rotational transitions of the observed molecules may have excitation
temperatures significantly lower than this value because of subthermalization,
i.e., the critical density of the transition is higher than the volume density of
the cloud,
13 K.
Yet, the rotational transitions of the observed molecules may have excitation
temperatures significantly lower than this value because of subthermalization,
i.e., the critical density of the transition is higher than the volume density of
the cloud,
![]() cm-3. In particular this is the case for the high
dipole moment molecules like HCO+ (3.90 Debyes), HCN (2.98 Debyes), H2CO
(2.33 Debyes) or CS (1.98 Debyes). Thus, for example, the critical density of
the H2CO 30,3-20,2 and CS 5-4 are 6
cm-3. In particular this is the case for the high
dipole moment molecules like HCO+ (3.90 Debyes), HCN (2.98 Debyes), H2CO
(2.33 Debyes) or CS (1.98 Debyes). Thus, for example, the critical density of
the H2CO 30,3-20,2 and CS 5-4 are 6
![]() and 7
and 7
![]() cm-3,
respectively (Choi & Zhou 1997).
cm-3,
respectively (Choi & Zhou 1997).
If at least two transitions are detected and the emission is optically thin,
the excitation temperature can be obtained from the following two expressions:
The beam-averaged column densities of the observed species were derived
assuming that the excitation temperature,
![]() ,
is the same for all the
rotational transitions of the same species, with the exception of the SO and
CH3OH, whose column densities were derived from the Population Diagram
(Sect. 3.1.1).
,
is the same for all the
rotational transitions of the same species, with the exception of the SO and
CH3OH, whose column densities were derived from the Population Diagram
(Sect. 3.1.1).
The HCO+ column density can be derived from the model carried out using the
Monte Carlo radiative transfer code (Sect. 3.1.2) or directly from the line
intensity ratios as described earlier (Eqs. (2) and (1)). From the
model derived in Sect. 3.1.2, the HCO+ beam-averaged column density for
the best fitted model is 1.0
![]() cm-2. On the other hand, for the intensity
ratio method, the filling factor ratio of the 4-3 and 3-2
transitions,
f21''/f28'', should be derived in order to properly use
Eqs. (2) and (1). From the FWHM of the HCO+ 1-0 emission,
34'' (see Sect. 3.1.2) we can obtain a rough estimate of this ratio:
cm-2. On the other hand, for the intensity
ratio method, the filling factor ratio of the 4-3 and 3-2
transitions,
f21''/f28'', should be derived in order to properly use
Eqs. (2) and (1). From the FWHM of the HCO+ 1-0 emission,
34'' (see Sect. 3.1.2) we can obtain a rough estimate of this ratio:
![]() .
Thus from the line ratio analysis we estimate
that the H13CO+ 3-2 transition is optically thin (
.
Thus from the line ratio analysis we estimate
that the H13CO+ 3-2 transition is optically thin (![]() )
and that
the excitation temperature is within the 6.2-7.0 K range. Therefore, from the
H13CO+ 3-2 integrated line intensity, the HCO+ beam-averaged column
density is within the 3-5
)
and that
the excitation temperature is within the 6.2-7.0 K range. Therefore, from the
H13CO+ 3-2 integrated line intensity, the HCO+ beam-averaged column
density is within the 3-5
![]() cm-2 range. The line intensity ratio
analysis derives an excitation temperature lower than the kinetic temperature
obtained in Sect. 3.1.2 and also a lower column density (by a factor 2-3).
Uncertainties in the
f21''/f28'' derived from the HCO+ 1-0 maps
(see Sect. 3.1.2) could account for this discrepancy.
Alternatively, non-LTE effects, due to subthermalization, could also cause
this discrepancy.
cm-2 range. The line intensity ratio
analysis derives an excitation temperature lower than the kinetic temperature
obtained in Sect. 3.1.2 and also a lower column density (by a factor 2-3).
Uncertainties in the
f21''/f28'' derived from the HCO+ 1-0 maps
(see Sect. 3.1.2) could account for this discrepancy.
Alternatively, non-LTE effects, due to subthermalization, could also cause
this discrepancy.
Since the DCO+ has a similar dipole moment as the HCO+, we adopted the
same excitation temperature derived from the line ratios of the HCO+: within
the 6-7 K range, the column density of the DCO+ column density is
![]() cm-2. This value is two orders of magnitude lower than that
of the HCO+.
cm-2. This value is two orders of magnitude lower than that
of the HCO+.
For the DCN, we adopted the same excitation temperature as the one for the HCN, 6 K.
The two transitions detected yield an excitation temperature of ![]() 6 K,
implying that the emission is subthermalized (the upper limits of the
undetected transitions are not tight enough to better constrain this value). As
in the case of HCN and HCO+ this is due to its high dipole moment. In order
to calculate the H2CO beam-averaged column density we used this excitation
temperature and adopted an ortho to para ratio of 1.5, which should be
reasonable for the H2CO formation and/or ortho-para conversion on grain
surfaces (Dickens & Irvine 1999).
6 K,
implying that the emission is subthermalized (the upper limits of the
undetected transitions are not tight enough to better constrain this value). As
in the case of HCN and HCO+ this is due to its high dipole moment. In order
to calculate the H2CO beam-averaged column density we used this excitation
temperature and adopted an ortho to para ratio of 1.5, which should be
reasonable for the H2CO formation and/or ortho-para conversion on grain
surfaces (Dickens & Irvine 1999).
![\begin{figure}
\par\includegraphics[width=7cm,clip]{MS2228f5.eps} \end{figure}](/articles/aa/full/2002/24/aa2228/Timg62.gif) |
Figure 5:
Excitation temperatures as a function of the dipole moment for the molecules
with two or more transitions detected towards the quiescent ambient cloud
ahead of HH 2. The
|
| Open with DEXTER | |
The excitation temperature derived from the two transitions is ![]() 8 K. This
slightly higher excitation temperature than that derived from the other
molecules is possibly due to its slightly lower dipole moment. As in the
previous cases, this excitation temperature was used to derive the column
density.
8 K. This
slightly higher excitation temperature than that derived from the other
molecules is possibly due to its slightly lower dipole moment. As in the
previous cases, this excitation temperature was used to derive the column
density.
Of the five SO2 transitions observed, only two transitions were detected,
31,3-20,2 and 60,6-51,5. If their emission is optically
thin, then their line ratio gives an unusually high excitation temperature,
![]() 280 K, much higher than that derived from other molecules. At this
temperature the 101,9-92,8 and 101,9-100,10 transitions
should have been detected with BIMA. Therefore, this temperature seems
unreliable. If an excitation temperature of about 10 K is assumed, then the
line ratio gives an 31,3-20,2 optical depth of
10. From the radiative transfer equation for the optically thick case,
280 K, much higher than that derived from other molecules. At this
temperature the 101,9-92,8 and 101,9-100,10 transitions
should have been detected with BIMA. Therefore, this temperature seems
unreliable. If an excitation temperature of about 10 K is assumed, then the
line ratio gives an 31,3-20,2 optical depth of
10. From the radiative transfer equation for the optically thick case,
![]() ,
the filling factor can be derived and,
therefore, a rough estimation of the source size,
,
the filling factor can be derived and,
therefore, a rough estimation of the source size, ![]() ,
,
![]() (30'' is the angular resolution of the spectra).
The peak intensity of the 31,3-20,2 line,
(30'' is the angular resolution of the spectra).
The peak intensity of the 31,3-20,2 line, ![]() 0.2 K, would imply
a source size of only 5'' if the line is optically thick. However, the BIMA
maps of this transition show that the emission arises from a significantly
larger area,
0.2 K, would imply
a source size of only 5'' if the line is optically thick. However, the BIMA
maps of this transition show that the emission arises from a significantly
larger area,
![]() (Paper II). Thus, we suggest that the
60,6-51,5 line emission is "anomalous'', although we cannot discern
if this is an instrumental effect or if it is a real anomaly. In order to
estimate the SO2 column density we used the 31,3-20,2 transition,
assuming an excitation temperature of 10 K.
(Paper II). Thus, we suggest that the
60,6-51,5 line emission is "anomalous'', although we cannot discern
if this is an instrumental effect or if it is a real anomaly. In order to
estimate the SO2 column density we used the 31,3-20,2 transition,
assuming an excitation temperature of 10 K.
The CO and its isotopes have a low dipole moment, 0.11 Debyes, so their
emission is likely thermalized. Therefore, the excitation temperature used to
derived the CO column density is 13 K.
Figure 5 shows the values of the excitation temperature versus the dipole moment of the analyzed aforementioned molecules. With the
exception of the HCO+, there is a trend of decreasing
![]() with increasing
dipole moment,
with increasing
dipole moment, ![]() ,
which is expected since higher
,
which is expected since higher ![]() implies more severe
subthermalization. Figure 5 can be used to roughly extrapolate
the excitation temperature for the other observed molecules. Thus, we adopted
a
implies more severe
subthermalization. Figure 5 can be used to roughly extrapolate
the excitation temperature for the other observed molecules. Thus, we adopted
a
![]() K for those molecules with
K for those molecules with ![]() Debyes (CN, OCS, H2S,
HCS+, H2CS, HNCO, HCOOH and CH3SH) and
Debyes (CN, OCS, H2S,
HCS+, H2CS, HNCO, HCOOH and CH3SH) and
![]() K for the rest of
the molecules (c-C3H2, HC3N, CH3CN and NH2CN), which indeed have a
dipole moment higher than 3 Debyes.
K for the rest of
the molecules (c-C3H2, HC3N, CH3CN and NH2CN), which indeed have a
dipole moment higher than 3 Debyes.
Table 3 shows the beam-averaged column densities derived from the
observations. The fractional abundances of the observed molecules with respect
to the CO molecule are also listed in this table (note that the estimated
fractional abundance relative to the CO is a beam-averaged value).
| Column | X[molecule]/ | |
| Molecule | Density | X[CO] |
| COa | 3.8
|
1.0 |
| 13CO | 6.0
|
1.6
|
| C18O | 6.8
|
1.8
|
| HCO+ | 1.0
|
2.6
|
| CH3OH | 6.4
|
1.7
|
| H2CO | 5.7
|
1.7
|
| SO | 3.2
|
8.4
|
| SO2 | 1.7
|
4.4
|
| HCN | 4.1
|
1.1
|
| CN | 3.4
|
8.9
|
| CS | 2.8
|
7.3
|
| DCN | 1.0
|
2.6
|
| DCO+ | 1.0
|
2.6
|
| HCS+ | ||
| c-C3H2 | ||
| HNCO | ||
| CH3CN | ||
| NH2CN | ||
| HC3N | ||
| H2CS | ||
| HCOOH | ||
| CH3SH | ||
| OCS | ||
| NS | ||
| H2S |
![\begin{figure}
\par\includegraphics[width=8.8cm,clip]{MS2228f6.eps} \end{figure}](/articles/aa/full/2002/24/aa2228/Timg81.gif) |
Figure 6: Relative molecular abundances (with respect to the CO) for HH 2 (filled circles), high mass star forming cores, low mass star forming cores and dark clouds (i.e., without star formation activity). The filled square within the bar and the bar show the logarithmic median and the logarithmic standard deviation, respectively, of the sample. The sample for the high mass star forming clouds is: W3 (Helmich & van Dishoeck 1997), M 17 and Cepheus A (Bergin et al. 1997). The sample for the low mass star forming molecular clouds is: IRAS 16293 (van Dishoeck et al. 1995), NGC 1333 IRAS4A (Blake et al. 1995), the Serpens S68 (McMullin et al. 2000), 05338-0624 (McMullin et al. 1994) and several other regions for H2CO (Dickens & Irvine 1999) and CH3OH (Kalenskii & Sobolev 1994). The sample for the quiescent dark molecular clouds is: OMC-1N (Ungerechts et al. 1997), TMC-1 (Pratap et al. 1997), L134N (Dickens et al. 2000), and the CB clouds for H2CO (Turner 1994). The references of several of the selected molecular clouds give the relative chemical abundances in different positions of the cloud, so most of the data plotted in this figure were obtained averaging four or more points (the SO2and CN values in low mass star forming cores are from only one region). |
| Open with DEXTER | |
In order to be able to establish the effects of the shock-induced radiation from HH 2 on the chemical composition of the quiescent clump ahead of the HH object, it is necessary to compare the relative abundances of the molecules detected with respect to other environments associated with dense molecular material. Before the radiation reached the clump, this molecular cloud had likely the properties of the starless dark molecular cores. It is well known that there are important chemical differences between starless dark clouds and star forming regions, and that there are important gradients within the same molecular cloud, a consequence of the evolutionary effects or due to the presence of nearby star formation sites (e.g., van Dishoeck & Blake 1998). Since we want to compare the general chemical properties of the molecular clouds with those from the molecular emission ahead of HH 2, we have estimated the logarithmic median and standard deviation of the relative abundance (with respect to CO) of the molecules detected in HH 2 and compare this with a sample of three different types of molecular environments: high mass and low mass star forming molecular clouds, and starless dark molecular clouds. Figure 6 shows shows this comparison.
Interestingly, from Fig. 6, the observed molecules ahead of HH 2 can be classified into four groups, depending on their abundance with respect to other molecular clouds. (1) Strongly enhanced molecules: HCO+, CH3OH and H2CO. Their relative abundances are enhanced mostly within a factor of 5 to 10 with respect to the typical values found in the molecular clouds. In particular, the enhancement of HCO+ and H2CO in HH 2 with respect to the high mass star forming regions is more than an order of magnitude, but only by a factor of 2-3 with respect to the dark molecular clouds. (2) Weakly enhanced molecules, SO and SO2. Their enhancement with respect to the molecular clouds is a factor of 2-4. The only exception is that the SO2 has similar relative abundances as the massive star forming regions. (3) The CN shows no apparent enhancement with respect to the dark molecular clouds, whereas it is depleted with respect to the high mass star forming regions. The strong enhancement (an order of magnitude) with respect to the low mass star forming regions may not be significant since there is only one data point. (4) Depleted molecules: CS and HCN. In HH 2 these molecules are clearly depleted with respect to the other molecular clouds, with depletion factors in the 3 to 7 range.
Here we briefly compare our observational results with the abundances derived
in the VW99 models. A more detailed model of the HH2 clump (in light of the
present observations) will be the subject of a future paper. Columns 3 to 6 of
Table 4 lists the best matched theoretical column densities and the
visual extinction at which this column density was obtained for two models out
of the grid from VW99 for clump densities of 1
![]() cm-3 (Model 4) and of
1
cm-3 (Model 4) and of
1
![]() cm-3 (Model 5), 3 years after irradiation of the clump has started.
cm-3 (Model 5), 3 years after irradiation of the clump has started.
Table 4 clearly confirms the qualitative results from VW99 where many species were predicted to be abundant in clumps ahead of HH objects. The VW99 models were not specific to any particular HH object but use an extensive gas and grain phase chemistry to follow the formation of a clump ahead of a shock with a fixed enhanced radiation field (equivalent to 20 times the ambient interstellar radiation field) acting upon it. The clump is treated as a one-dimensional slab extending up to 6 visual magnitudes to the center.
| HH 2 | Model 4 | Model 5 | ||||
| Molecule |
|
|
|
|||
| CO | 3.8
|
3.1
|
3.2 | 3.2
|
3.1 | |
| HCO+ | 1.0
|
3
|
1.0 | 2.5
|
1.1 | |
| CH3OH | 6.4
|
7.8
|
1.3 | 4.0
|
1.1 | |
| H2CO | 5.7
|
3.3
|
6.0 | 5.7
|
2.0 | |
| SO | 3.2
|
3.0
|
3.4 | 5.7
|
2.8 | |
| SO2 | 1.7
|
1.8
|
4.1 | 1.9
|
3.8 | |
| HCN | 4.1
|
4-5
|
1-2 | 5.0
|
1.3 | |
| CN | 3.4
|
4
|
1.7 | 3.4
|
1.3 | |
| CS | 2.8
|
8
|
1.6 | 5.0
|
1.9 | |
In general, the VW99 models suggest that: (i) HH2 is a young object, probably
on the order of 1000 years; (ii) the best estimate for the volume density of
the clump is <105 cm-3, somewhat less than the density derived from
observations; (iii) the visual extinction at the center of the SO peak emission
with respect to HH 2 is about 5-7 mags; VW99 models only extend to 6 mag;
although quantitatively the models do not match the observed abundances for
every species at the maximum ![]() ,
most of them are well represented at
some visual extinction. In fact, Model 4 of VW99 reproduces the observed
abundance (within half order of magnitude) for every species for an optimal
visual extinction, different from species to species: this may be an indication
of the displacement found with the BIMA array (Paper II). More specifically, it
is interesting to note that HCO+, the best tracer of HH clumps, is
underabundant for visual extinction larger than
,
most of them are well represented at
some visual extinction. In fact, Model 4 of VW99 reproduces the observed
abundance (within half order of magnitude) for every species for an optimal
visual extinction, different from species to species: this may be an indication
of the displacement found with the BIMA array (Paper II). More specifically, it
is interesting to note that HCO+, the best tracer of HH clumps, is
underabundant for visual extinction larger than ![]() 1 mag. This is because
at high visual extinction the clump is more shielded from the radiation and
this would limit the amount of ionized carbon, yielding a low production of
HCO+; a higher radiation field may well therefore increase its abundance.
The presence of X-ray emission in HH 2 (Pravdo et al. 2001) may have
some effects on the chemistry. A detailed model for HH 2 which takes such
considerations into account is in preparation.
1 mag. This is because
at high visual extinction the clump is more shielded from the radiation and
this would limit the amount of ionized carbon, yielding a low production of
HCO+; a higher radiation field may well therefore increase its abundance.
The presence of X-ray emission in HH 2 (Pravdo et al. 2001) may have
some effects on the chemistry. A detailed model for HH 2 which takes such
considerations into account is in preparation.
We presented a 3-0.8 mm molecular line survey toward the quiescent molecular
clump ahead of the bright HH 2 object. A total of 14 species were detected
(including different isotopes and deuterated species) and the upper limits of
12 more species were obtained. Multi-transitions observations of HCO+, SO and
CH3OH show that the gas is not only quiescent, but also cold, ![]() 13 K,
in spite of being close to HH 2. The density of the cloud is roughly
3
13 K,
in spite of being close to HH 2. The density of the cloud is roughly
3![]() cm-3. Comparisons of the relative abundances (with respect to CO)
were made with different dense molecular gas environments, including quiescent,
starless dark clouds, and active low and high mass star forming cores. We
confirm the peculiar chemical composition of the gas ahead of the HH objects, a
consequence of the mantle removal and further photochemistry produced by the
strong radiation generated in the shocks. In particular, we found that:
cm-3. Comparisons of the relative abundances (with respect to CO)
were made with different dense molecular gas environments, including quiescent,
starless dark clouds, and active low and high mass star forming cores. We
confirm the peculiar chemical composition of the gas ahead of the HH objects, a
consequence of the mantle removal and further photochemistry produced by the
strong radiation generated in the shocks. In particular, we found that:
Acknowledgements
We thank S. Curiel for providing the [SII] images of HH 2. We thank W. Dent for his valuable comments. JMG acknowledge support by NSF grant AST-99-81363 and by RED-2000 from the Generalitat de Catalunya. RE and JMG are partially supported by DGICYT grant PB98-0670 (Spain). SV and DAW thank PPARC for supporting their research.
The population diagram (also called rotation diagram in the literature)
analysis has been extensively used in the literature to obtain the temperature
and the column densities for molecules with several transitions observed (e.g.,
Linke et al. 1979; Turner 1991). In the
use of the population diagram, we assume LTE conditions (i.e., all the
transitions are populated with a single excitation temperature
![]() ),
optically thin emission and
),
optically thin emission and
![]() .
Under these assumptions the
population diagram can be described with the following expression:
.
Under these assumptions the
population diagram can be described with the following expression:
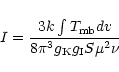 |
(A.2) |
In order to better use the population diagram technique we just assume that all
the rotational levels are populated with the same excitation temperature (which
in most cases is likely lower than the kinetic temperature: see Goldsmith &
Langer 1999). In this case the expression is:
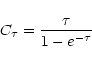 |
(A.4) |
![\begin{displaymath}F_{\rm T} = \frac{J_{\nu}[T_{\rm ex}]}{J_{\nu}[T_{\rm ex}]-J_{\nu}[T_{\rm bg}]}
\end{displaymath}](/articles/aa/full/2002/24/aa2228/img97.gif) |
(A.5) |
The optical depth for each transition can be estimated if true column
density,
![]() ,
is known. Since
,
is known. Since
![]() ,
the optical depth of a given rotational transition is given by:
,
the optical depth of a given rotational transition is given by:
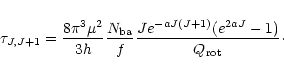 |
(A.6) |
To estimate the best set of solutions we calculated the ![]() value for each
set of parameters,
value for each
set of parameters,
![]() ,
,
![]() and f, taking into
account the uncertainties (rms noise and calibration errors) in a similar way
as that described by Nummelin et al. (1998) and Gibb et al. (2000):
and f, taking into
account the uncertainties (rms noise and calibration errors) in a similar way
as that described by Nummelin et al. (1998) and Gibb et al. (2000):
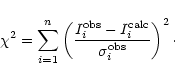 |
(A.7) |