A&A 365, 241-247 (2001)
DOI: 10.1051/0004-6361:20000026
D. Chastaing - S. D. Le Picard - I. R. Sims - I. W. M. Smith
Send offprint request: I. W. M. Smith,
School of Chemistry, The University of Birmingham, Edgbaston, Birmingham B15 2TT, UK
Received 29 August 2000 / Accepted 11 October 2000
Abstract
Rate coefficients for the reactions of ground state carbon atoms, C(3PJ), with the
four unsaturated hydrocarbons C2H2, C2H4,
![]() and
and
![]() have been measured
at temperatures down to 15 K. The experiments have been performed in a continuous flow
CRESU (Cinétique de Réaction en Écoulement Supersonique Uniforme) apparatus using
pulsed laser photolysis of C3O2 to generate C(3PJ) atoms and laser-induced fluorescence in the
vacuum ultraviolet to observe the kinetic decays of the atoms and hence determine the rate
coefficients. All four reactions are found to occur at rates close to the collision-determined
limit and the rates show at most a very mild dependence on temperature. It is argued that
normally these reactions proceed along a reaction path with no barrier to form a strongly
bound energised adduct, which subsequently eliminates an H atom to form the reaction
products. The rates of the reactions of C(3PJ) atoms with larger unsaturated hydrocarbons are
discussed. Finally, the relevance of the results for the chemistry of dense interstellar clouds is
considered.
have been measured
at temperatures down to 15 K. The experiments have been performed in a continuous flow
CRESU (Cinétique de Réaction en Écoulement Supersonique Uniforme) apparatus using
pulsed laser photolysis of C3O2 to generate C(3PJ) atoms and laser-induced fluorescence in the
vacuum ultraviolet to observe the kinetic decays of the atoms and hence determine the rate
coefficients. All four reactions are found to occur at rates close to the collision-determined
limit and the rates show at most a very mild dependence on temperature. It is argued that
normally these reactions proceed along a reaction path with no barrier to form a strongly
bound energised adduct, which subsequently eliminates an H atom to form the reaction
products. The rates of the reactions of C(3PJ) atoms with larger unsaturated hydrocarbons are
discussed. Finally, the relevance of the results for the chemistry of dense interstellar clouds is
considered.
Key words: molecular data - molecular processes - methods: laboratory - ISM: clouds
Author for correspondance: i.w.m.smith@bham.ac.uk
Carbon is the fourth commonest element in the universe with an abundance of ca. 3 10-4
relative to that of hydrogen (Anders & Grevesse 1989; Cardelli et al. 1996). Ground
state carbon atoms, C(3PJ), have been detected in a wide variety of astronomical environments.
They are particularly abundant in dense interstellar clouds (ISCs) (Phillips & Huggins 1981;
Schilke et al. 1995; Zmuidzinas et al. 1988), where their abundance is comparable
to that of CO, the second commonest molecule in ISCs. The typical column density of carbon atoms is
![]() (Phillips & Huggins 1981; Schilke
et al. 1995; Zmuidzinas et al. 1988; Petitpas & Wilson 1998).
C(3PJ) atoms have also been detected in the circumstellar envelopes of evolved stars, such as the outer shell of
IRC
(Phillips & Huggins 1981; Schilke
et al. 1995; Zmuidzinas et al. 1988; Petitpas & Wilson 1998).
C(3PJ) atoms have also been detected in the circumstellar envelopes of evolved stars, such as the outer shell of
IRC
![]() and the inner envelope of
and the inner envelope of ![]() Ori (Van der Veen et al. 1998). Of the
approximately 120 molecules that have been definitely identified in ISCs (Herbst 1996), over two-thirds
contain carbon and most of these are "carbon-rich'', despite the overwhelmingly high abundance of hydrogen in such
clouds.
Ori (Van der Veen et al. 1998). Of the
approximately 120 molecules that have been definitely identified in ISCs (Herbst 1996), over two-thirds
contain carbon and most of these are "carbon-rich'', despite the overwhelmingly high abundance of hydrogen in such
clouds.
One of the aims of astrophysical chemists is to understand the chemistry that leads to the observed abundances of molecules in dense ISCs. To this end, reaction networks have been constructed and computer simulations of the molecular evolution in dense ISCs have been carried out. In recent years, the importance of reactions between electrically neutral species has been recognised (Herbst et al. 1994; Sims & Smith 1996), even though the temperatures in dense ISCs are typically between 10 and 50 K. One problem is how larger molecules are assembled and, in particular, how molecules containing chains of several carbon atoms are created.
Experiments from our laboratory, including many in collaboration with Rowe's group
from Rennes, have demonstrated that the radicals CH (Canosa et al. 1997) and C2H
(Chastaing et al. 1998) react rapidly with unsaturated hydrocarbons at low temperatures. It
has been argued that the reaction mechanism probably involves the incorporation of one
(with CH) or two (with C2H) more carbon atoms into the molecule and the elimination of a
hydrogen atom; i.e.
| (1) |
Neutral-neutral reactions of atomic carbon with unsaturated hydrocarbons have been
identified by Herbst and co-workers (Herbst et al. 1994; Bettens et al. 1995;
Bettens & Herbst 1997) as potentially of great importance in the synthesis of larger carbon-containing
species, if successive addition-elimination reactions occur, similar to those represented by
Eq. (1); i.e.
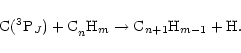 |
(2) |
The only kinetic data for reactions of C(3PJ) atoms with unsaturated hydrocarbons at temperatures below room temperature are the earlier measurements from our laboratory (Chastaing et al. 1999). Those experiments, like the present ones, employed the CRESU technique (CRESU is a French acronym for Reaction Kinetics in Uniform Supersonic Flow). This technique relies on the cooling that occurs when gas is expanded through a convergent-divergent Laval nozzle to generate a supersonic flow of relatively dense gas at a defined temperature. Kinetic measurements on reactions involving neutral radicals, including radical atoms, are then normally made by: (i) generating the radicals by pulsed laser photolysis of a suitable precursor, and (ii) observing subsequent changes in the concentration of the radicals, using an appropriate spectroscopic technique (Sims & Smith 1996). In the present experiments, as in our earlier ones, C(3PJ) atoms were generated by photolysis of C3O2 using the output of an ArF excimer laser at 193 nm. In the earlier experiments, the subsequent kinetic behaviour of these atoms was inferred by observing chemiluminescence from NO formed in the reaction between the C(3PJ) atoms and a small concentration of NO2 that was included in the reaction mixture. In the present experiments, we have observed decays in the concentration of C(3PJ) atoms directly by applying the technique of time-resolved laser-induced fluorescence (LIF) in the vacuum ultraviolet (VUV). Further details of this method are given in the next section.
The rate constants for the reactions of C(3PJ) atoms with unsaturated hydrocarbons have been
measured in the Birmingham CRESU apparatus (James et al. 1998) at temperatures down to
15 K. In a CRESU apparatus, low temperatures are achieved by isentropic expansion of a gas
mixture, consisting predominantly (typically ![]() )
of a carrier gas (He, Ar or N2) through
an axisymmetric, convergent-divergent, Laval nozzle. A supersonic flow of gas is generated
in which the Mach number, the temperature, the total pressure of the gas, and the mole
fractions of the components of the mixture are uniform along the flow. In all the experiments
referred to here, a small concentration of C3O2 was included in the flow and C(3PJ) atoms
were generated by pulsed laser photolysis of this precursor at 193 nm, using an excimer laser
operating on ArF. The output from this laser was directed through the gas reservoir and Laval
nozzle and along the axis of the gas flow.
)
of a carrier gas (He, Ar or N2) through
an axisymmetric, convergent-divergent, Laval nozzle. A supersonic flow of gas is generated
in which the Mach number, the temperature, the total pressure of the gas, and the mole
fractions of the components of the mixture are uniform along the flow. In all the experiments
referred to here, a small concentration of C3O2 was included in the flow and C(3PJ) atoms
were generated by pulsed laser photolysis of this precursor at 193 nm, using an excimer laser
operating on ArF. The output from this laser was directed through the gas reservoir and Laval
nozzle and along the axis of the gas flow.
In the present CRESU experiments, data for the reactions of C(3PJ) atoms have been obtained by directly monitoring the kinetic decays of the C(3PJ) atomic concentration using resonant vacuum-ultraviolet laser-induced fluorescence (VUV-LIF); that is, with both excitation and observation of the fluorescence on the (2s22p3s 3PJ - 2s22p2 3PJ) transition in atomic carbon. Pulses of VUV laser radiation were generated using four-wave mixing in Xe (Hilbig & Wallenstein 1983a). The method is described in some detail elsewhere (Chastaing et al. 2000a). Briefly, dye laser radiation was tuned to 255.94 nm in order to excite Xe in a two-photon transition to its 5p56p[21/2, 2] state. A second pulsed dye laser could be tuned through the wavelength range between 560 and 565 nm to generate VUV radiation between 165.9 and 165.4 nm. This radiation propagated horizontally and was directed perpendicular to the gas flow at a fixed point in the apparatus. LIF spectra of the (2s22p3s 3PJ - 2s22p2 3PJ) transition were recorded and confirmed that spin-orbit relaxation within the electronic ground state was achieved on a time-scale that was short relative to that for reaction (Chastaing et al. 2000a; Canosa et al. 2000).
For kinetic measurements, the VUV probe laser was set at the wavelength of the (2s22p3s
3P
J=1 - 2s22p2 3PJ=0) transition at 165.69 nm and LIF signals, observed by a
photomultiplier mounted above the gas flow, were recorded as the time delay between the pulses from the
photolysis and probe lasers was systematically varied. The recorded traces of LIF signal
versus time accurately fitted single exponential decays yielding pseudo-first-order rate
constants (
![]() ), as long as sufficient delay (ca. 10
), as long as sufficient delay (ca. 10 ![]() s) was allowed before starting the fitting to
allow for complete relaxation among the spin-orbit components of the C(3PJ) ground state.
Second-order rate constants for reaction at a particular temperature were obtained by
measuring values of
s) was allowed before starting the fitting to
allow for complete relaxation among the spin-orbit components of the C(3PJ) ground state.
Second-order rate constants for reaction at a particular temperature were obtained by
measuring values of
![]() ,
with different concentrations of the co-reagent (CnHm) added to the
flowing gas mixture, and plotting
,
with different concentrations of the co-reagent (CnHm) added to the
flowing gas mixture, and plotting
![]() versus [CnHm].
versus [CnHm].
C3O2 was synthesised by the dehydration of bis(trimethylsilyl) malonate as described by
Chastaing et al. (1999). C2H2, C2H4, and the carrier gases (He, Ar and N2) were provided
by Air Products. Methyl acetylene (or propyne; i.e. CH
![]() )
and allene (or propadiene; i.e.
)
and allene (or propadiene; i.e.
![]() )
were supplied by Intergas. All gases were used directly from their cylinders
without further purification.
)
were supplied by Intergas. All gases were used directly from their cylinders
without further purification.
The rate coefficients for the reactions of C(3PJ) atoms with the unsaturated hydrocarbons,
C2H2, C2H4,
![]() and
and
![]() ,
which have been determined in the present work
are listed in Table 1, along with the main flow conditions for each measurement.
,
which have been determined in the present work
are listed in Table 1, along with the main flow conditions for each measurement.
| Temperature (K) | 15 | 27 | 54 | 83 | 207 | 295 |
| Carrier gas | He | He | Ar | N2 | N2 | Ar |
| Total density | ||||||
| (1016 mol cm-3) | 5.05 | 4.65 | 5.36 | 4.88 | 5.83 | 19-20 |
| Range of reagent gas | ||||||
| (1013 mol cm-3) | ||||||
| C2H2 | 0-5.9 | 0-5.7 | 0-7.7 | 0-10 | 0-7.0 | 0-57 |
| C2H4 | 0-5.8 | 0-5.5 | 0-7.4 | 0-14 | 0-6.7 | 0-60 |
| CH3C |
0-0.82 | 0-3.5 | 0-4.2 | 0-3.7 | 0-4.8 | 0-50.8 |
|
|
0-1.9 | 0-4.0 | 0-4.9 | 0-3.8.9 | 0-4.9 | 0-23.6 |
| Rate coefficient | ||||||
| (10-10 cm3 mol-1 s-1) | ||||||
| C2H2 |
|
|
|
|
|
|
| C2H4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 1 Estimated errors are quoted
|
||||||
| t-distribution for 95% confidence. |
In Figs. 1 and 2, the rate coefficients, k(T), that we have determined for the reactions of
C(3PJ) atoms with C2H2 and C2H4 are displayed as a function of temperature (T) on log-log
plots.
![\begin{figure}
\par\includegraphics[width=7.7cm,clip]{aa10229f1.eps} \end{figure}](/articles/aa/full/2001/02/aa10229/Timg48.gif) |
Figure 1:
Rate coefficients for the reaction of C(3PJ) atoms with C2H2 as a function of
temperature plotted on a log-log scale. The filled circles ( |
| Open with DEXTER | |
![\begin{figure}
\par\includegraphics[width=7.7cm,clip]{aa10229f2.eps} \end{figure}](/articles/aa/full/2001/02/aa10229/Timg49.gif) |
Figure 2:
Rate coefficients for the reaction of C(3PJ) atoms with C2H4 as a function of
temperature plotted on a log-log scale. The symbols have the same meaning as in Fig. 1.
The line is a fit to all the CRESU data yielding:
|
| Open with DEXTER | |
| Reaction | A / 10-10 cm3 mol-1 s |
na |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| a Errors are quoted as
|
In Figs. 1 to 2, we have also plotted the rate constants obtained in previous studies of the
reactions of C(3PJ) atoms with C2H2 and C2H4. Except for the earlier results which we
obtained for these reactions using the chemiluminescent marker technique, the previous data
are limited to room temperature. The present results are in excellent agreement with those
obtained using the chemiluminescent technique. The parameters A and n found by fitting both
sets of data for each reaction, according each set equal weighting, are also listed in Table 2.
Our results at room temperature are in fair agreement with those of Haider & Husain (1993).
For
![]() and
and
![]() ,
the only previous kinetic
data are the room temperature rate constants of Haider & Husain (1993). The rate coefficients that they
reported are 60% larger than ours in the case of
,
the only previous kinetic
data are the room temperature rate constants of Haider & Husain (1993). The rate coefficients that they
reported are 60% larger than ours in the case of
![]() and 2.3 times larger for
and 2.3 times larger for
![]() .
.
For all four reactions that we have studied here, there is no apparent dependence on the nature of the carrier gas confirming that the experiments determine rate coefficients for bimolecular reactions between the unsaturated hydrocarbon and the C(3PJ) atoms.
The present measurements confirm our earlier conclusion (Chastaing et al. 1999) that C(3PJ)
atoms react rapidly with simple unsaturated hydrocarbons at very low temperatures. The
rapidity of these reactions, and the fact that their rate coefficients are almost independent of
temperature leads to two important fundamental conclusions. First, there must be no barrier
on the potential energy surfaces leading from reagents to products for these reactions. This
conclusion is confirmed by very recent crossed molecular beam experiments performed at low
collision energies on the reactions of C(3PJ) atoms with C2H2, C2H4,
![]() and
and
![]() (Chastaing et al. 2000b; Costes 2000). The cross-sections for all four of these reactions were shown
to increase monotonically as the relative translational energy was reduced from ca. 120 meV
to ca. 4 meV.
(Chastaing et al. 2000b; Costes 2000). The cross-sections for all four of these reactions were shown
to increase monotonically as the relative translational energy was reduced from ca. 120 meV
to ca. 4 meV.
In addition, it appears that the rate coefficients of these reactions of C(3PJ) atoms must be essentially independent of the spin-orbit state of the atom, i.e. of J. The spin-orbit states in C(3PJ) are closely spaced with J = 1 only 16.4 cm-1 (corresponding to 23.6 K) above the lowest J = 0 component, and J = 2 lying 43.4 cm-1 (corresponding to 62.4 K) above J = 0. There is a dramatic change in the relative spin-orbit populations as the temperature is reduced between 295 K (where NJ=0 : NJ=1 : NJ=2 = 1.0 : 2.77 : 4.05) and 15 K (where NJ=0 : NJ=1 : NJ=2 = 1.0 : 0.62 : 0.08). Consequently, if the rate coefficient for reactions of C(3PJ) atoms depended appreciably on the J level, then we should expect to have observed a similarly strong dependence of the thermally-averaged rate coefficients on temperature.
Our experimental results provide no information about the products of the reactions of
C(3PJ) atoms with C2H2, C2H4,
![]() and
and
![]() at low temperatures.
Of course, for modelling purposes, as well as for a full fundamental understanding of these reactions, it
is desirable to know the product branching ratios, as well as rate coefficients for the overall
reactions. Extensive quantum chemical calculations performed by Kaiser and co-workers
(Kaiser et al. 1996a; Kaiser et al. 1996b; Oschenfeld et al.
1997; Kaiser et al. 1997a; Kaiser et al. 1997b; Kaiser et al.
1999; Mebel et al. 2000) indicate that each of these reactions proceeds via the
initial formation of an adduct across an attractive potential energy surface with no barrier on the path leading from
reagents to the deep potential well associated with the adduct. In molecular beam experiments, and at the low total
pressures in interstellar clouds and in our experiments, the energised adduct will not be collisionally stabilised but
must fragment to yield two products. In some cases, fragmentation may occur at competitive rates to more than
one final set of products. However, in most cases the dominant process is likely to be loss of
an H atom, which is usually highly exothermic and requires the cleavage of just one C-H
bond.
at low temperatures.
Of course, for modelling purposes, as well as for a full fundamental understanding of these reactions, it
is desirable to know the product branching ratios, as well as rate coefficients for the overall
reactions. Extensive quantum chemical calculations performed by Kaiser and co-workers
(Kaiser et al. 1996a; Kaiser et al. 1996b; Oschenfeld et al.
1997; Kaiser et al. 1997a; Kaiser et al. 1997b; Kaiser et al.
1999; Mebel et al. 2000) indicate that each of these reactions proceeds via the
initial formation of an adduct across an attractive potential energy surface with no barrier on the path leading from
reagents to the deep potential well associated with the adduct. In molecular beam experiments, and at the low total
pressures in interstellar clouds and in our experiments, the energised adduct will not be collisionally stabilised but
must fragment to yield two products. In some cases, fragmentation may occur at competitive rates to more than
one final set of products. However, in most cases the dominant process is likely to be loss of
an H atom, which is usually highly exothermic and requires the cleavage of just one C-H
bond.
Table 3 lists the changes in enthalpy associated with (a) formation of the adduct between
C(3PJ) and the unsaturated hydrocarbon reagent, and (b) some of the more likely exothermic
channels identified in the theoretical work of Kaiser et al.
| Reagents | Products |
|
|
|
(HCCCH) |
|
| l-
|
|
|
| c-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| c-
|
|
|
| c-
|
|
|
|
|
|
|
| n-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| n-
|
|
|
|
|
|
|
|
|
|
The
![]() reaction was studied at three collision energies, 8.8, 28.0 and
45.0 kJ mol-1. They observed the angular distribution of products with m/e = 37, corresponding to C3H, and
were unable to detect any other reaction products (Kaiser et al. 1995; Kaiser et al.
1997a). From changes in the angular scattering with collision energy, they
inferred that both l-
reaction was studied at three collision energies, 8.8, 28.0 and
45.0 kJ mol-1. They observed the angular distribution of products with m/e = 37, corresponding to C3H, and
were unable to detect any other reaction products (Kaiser et al. 1995; Kaiser et al.
1997a). From changes in the angular scattering with collision energy, they
inferred that both l-
![]() and c-
and c-
![]() were formed under the conditions of their experiments, with the
contribution of the latter, more exothermic channel decreasing at higher collision energies. Of course, the collision
energies in Kaiser's experiments were appreciably larger than the average energy of thermal collisions between 10 and
50 K, that is at the temperatures typical of dense interstellar clouds, and the collision energies were also larger
than the exoergicities of the reactions to l-
were formed under the conditions of their experiments, with the
contribution of the latter, more exothermic channel decreasing at higher collision energies. Of course, the collision
energies in Kaiser's experiments were appreciably larger than the average energy of thermal collisions between 10 and
50 K, that is at the temperatures typical of dense interstellar clouds, and the collision energies were also larger
than the exoergicities of the reactions to l-
![]() and c-
and c-
![]() .
As the temperature is lowered,
one might expect the more exothermic channel producing c-
.
As the temperature is lowered,
one might expect the more exothermic channel producing c-
![]() to be increasingly favoured, so the
branching ratio between these two sets of products may change appreciably with temperature.
However, very recent calculations by Buonomo and Clary (Buonomo & Clary 2000)
performed on a new ab-initio surface have indicated that c-
to be increasingly favoured, so the
branching ratio between these two sets of products may change appreciably with temperature.
However, very recent calculations by Buonomo and Clary (Buonomo & Clary 2000)
performed on a new ab-initio surface have indicated that c-
![]() is disfavoured at low
temperatures: indeed, they predict negligible formation of the cyclic isomer at temperatures
below ca. 500 K.
is disfavoured at low
temperatures: indeed, they predict negligible formation of the cyclic isomer at temperatures
below ca. 500 K.
The production of both
![]() -
-
![]() and
and
![]() c-
c-
![]() from
from
![]() is spin-allowed. On the
other hand, the production of
is spin-allowed. On the
other hand, the production of
![]() which is much more
exothermic does not conserve spin. In their experiments, Kaiser et al. (1995, 1997b)
detected no reaction products other than
which is much more
exothermic does not conserve spin. In their experiments, Kaiser et al. (1995, 1997b)
detected no reaction products other than
![]() .
On the other hand, very recent flow-tube experiments by
Loison et al. (2000) at room temperature suggest that the yield of H atoms per C atom consumed in the
reaction between C(3PJ) atoms and C2H2 is no more than
.
On the other hand, very recent flow-tube experiments by
Loison et al. (2000) at room temperature suggest that the yield of H atoms per C atom consumed in the
reaction between C(3PJ) atoms and C2H2 is no more than
![]() .
It may be that, in this particular
reaction, as the loss of an H atom from the adduct is only slightly exothermic, the adduct is quite long-lived,
allowing time for intersystem crossing to a singlet surface, and subsequent production of C3(
.
It may be that, in this particular
reaction, as the loss of an H atom from the adduct is only slightly exothermic, the adduct is quite long-lived,
allowing time for intersystem crossing to a singlet surface, and subsequent production of C3(
![]() ,
to take place.
,
to take place.
For
![]() ,
the addition-elimination reaction to produce
,
the addition-elimination reaction to produce
![]() is more
exothermic (
is more
exothermic (
![]() kJ mol-1) than the corresponding channel in the reaction of C(3PJ)
atoms with C2H2. Therefore the energised adduct is likely to eliminate an H atom rapidly and
thereby suppress the contribution of any competing channels. In their crossed beam study of
the
kJ mol-1) than the corresponding channel in the reaction of C(3PJ)
atoms with C2H2. Therefore the energised adduct is likely to eliminate an H atom rapidly and
thereby suppress the contribution of any competing channels. In their crossed beam study of
the
![]() reaction, Kaiser et al. (1996) observed mass
39, consistent with the pathway to
reaction, Kaiser et al. (1996) observed mass
39, consistent with the pathway to
![]() ,
and Loison et al. (2000)
estimate the yield of H atoms from
,
and Loison et al. (2000)
estimate the yield of H atoms from
![]() to be
to be
![]() at
room temperature; that is, unity within their error limits. For the reactions of
C(3PJ) with both
at
room temperature; that is, unity within their error limits. For the reactions of
C(3PJ) with both
![]() and
and
![]() ,
observation of
products with mass 51 in crossed beam experiments (Kaiser et al. 1996b;
Kaiser et al. 1999), confirms the formation of C4H3 species. Again
the substitution of C for H, is strongly exothermic (see Table 3) and the
fragmentation of the initial adduct will probably be sufficiently rapid to suppress any other pathways.
,
observation of
products with mass 51 in crossed beam experiments (Kaiser et al. 1996b;
Kaiser et al. 1999), confirms the formation of C4H3 species. Again
the substitution of C for H, is strongly exothermic (see Table 3) and the
fragmentation of the initial adduct will probably be sufficiently rapid to suppress any other pathways.
In summary, at temperatures down as low as 15 K, C(3PJ) atoms react very rapidly with
the simple alkynes and alkenes, C2H2, C2H4,
![]() and
and
![]() .
With the
possible exception of the
.
With the
possible exception of the
![]() reaction, the products are very probably an H atom plus a
species derived by replacement of one H atom in the molecular reagent by a C atom.
reaction, the products are very probably an H atom plus a
species derived by replacement of one H atom in the molecular reagent by a C atom.
Of the alkynes and alkenes whose reactions with C(3PJ) atoms have been studied in the
present work, C2H2, C2H4 and
![]() have been positively identified in interstellar clouds.
Other such molecules that have been seen in ISCs include C4H2 and C6H2, i.e. di- and tri-
acetylene. Our measurements demonstrate that the first three of these molecules react at
essentially the collision-determined limit with C(3PJ) atoms at the temperatures found in dense
ISCs. At 25 K, a median temperature in such environments, the rate coefficients for all four of
the reactions studied in the present work, and the rate coefficient for
have been positively identified in interstellar clouds.
Other such molecules that have been seen in ISCs include C4H2 and C6H2, i.e. di- and tri-
acetylene. Our measurements demonstrate that the first three of these molecules react at
essentially the collision-determined limit with C(3PJ) atoms at the temperatures found in dense
ISCs. At 25 K, a median temperature in such environments, the rate coefficients for all four of
the reactions studied in the present work, and the rate coefficient for
![]() determined earlier by Chastaing et al. (1999) are the same within experimental uncertainty,
having values in the range 3.6-4.0
determined earlier by Chastaing et al. (1999) are the same within experimental uncertainty,
having values in the range 3.6-4.0
![]() cm3 mol-1 s-1.
cm3 mol-1 s-1.
The rate coefficients for reactions of radicals with alkenes and alkynes are sometimes correlated with properties of the reactants. For example, in the case of H atoms reacting with a series of alkenes, the activation energies associated with the reactions fall as the ionisation energies of the unsaturated hydrocarbons become smaller (Clarke et al. 2000 and references therein). Because the ionisation energies fall and the van der Waals attraction increases as the size of unsaturated hydrocarbons increases, there is every reason to suppose that the reactions of C(3PJ) atoms with larger alkenes and alkynes will be no smaller than those which we have determined directly for the simplest alkenes and alkynes.
Our measurements indicate that the reactions of C(3PJ) atoms with alkenes and alkynes are
rapid at low temperatures and should be included in chemical models of ISCs. It is argued
above that it is likely that, as with the reaction of CH and C2H with alkenes and alkynes,
reaction generally involves the addition of the atom or radical to the molecule and the loss of
an H atom. In this context, it is interesting to note that the radicals C3H, C5H and C7H have
all been observed in dense ISCs. These species could all be formed from reaction of C(3PJ)
atoms with the appropriate alkyne according to Eq. (2), i.e.
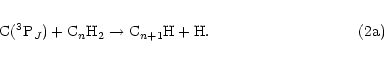
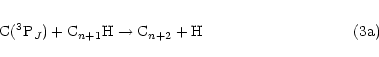
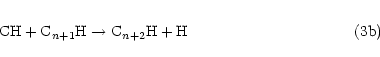
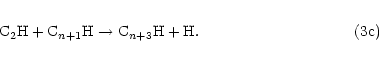
Acknowledgements
We thank the EPSRC for a substantial research grant to construct the CRESU apparatus, and for the loan of lasers through the Laser Loan Pool at the Rutherford-Appleton laboratory. We are grateful to the European Community for a TMR Research Network grant, contract FMRX-CT97-0132 (DG12-MIHT), for research on Astrophysical Chemistry under whose programme these experiments were performed and which provided a research Fellowship for S.D.L.P. We are also grateful to the TMR programme of the European Community for a research studentship for D. C. (contract ERBFMBICT961858). We thank Dr. Astrid Bergeat and Prof. David Clary for permission to cite their results (Loison et al. 2000; Buonomo & Clary 2000) prior to publication.