Table 3:
From the table containing all the emission lines found in
the spectrum, available at the CDS. The first column is the
wavelength in angstrom; the second column the relative peak
intensity; the third column contains the molecule name (or "Unid''
when the line is unidentified); electronic, vibrational and
rotational transitions are given in the last columns. In the list
we have included many blended lines noted with a " + '' sign.
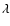 (Å)
(Å) |
Peak |
Species |
Electronic |
Vibrational |
Rotational |
| |
intensity |
|
transition |
transition |
transition |
| 6513.120 |
1.291 |
Unid |
|
|
|
| 6513.468 |
2.262 |
Unid |
|
|
|
| 6513.950 |
2.037 |
NH2 |
X-X |
(0, 12, 0)-(0, 0, 0) |
1 1 0-2 2 1 |
| + |
|
NH2 |
X-X |
(0, 12, 0)-(0, 0, 0) |
1 1 0-2 2 1 |
| 6514.140 |
1.110 |
C2 |
Swan |
4-7 |
R2( 6) |
| 6514.779 |
1.171 |
C2 |
Swan |
4-7 |
P2(27) P3(26) R3( 5) |
| 6514.920 |
1.248 |
Unid |
|
|
|
| 6515.145 |
1.162 |
C2 |
Swan |
4-7 |
R1( 6) |
| 6517.271 |
1.120 |
Unid |
|
|
|
| 6517.469 |
2.239 |
NH2 |
X-X |
(0, 12, 0)-(0, 0, 0) |
1 1 1-2 2 0 |
| 6517.730 |
1.110 |
NH2 |
X-X |
(0, 12, 0)-(0, 0, 0) |
1 1 1-2 2 0 |
| 6518.377 |
1.759 |
NH2 |
X-X |
(0, 12, 0)-(0, 0, 0) |
1 1 1-2 2 0 |
| 6519.489 |
1.707 |
NH2 |
X-X |
(0, 12, 0)-(0, 0, 0) |
2 1 1-3 2 2 |
Source LaTeX |
All tables |
In the text