All Tables
- Table 1:
A numerical example showing the mass-dependent
relations between the different reaction rates in the oxygen isotopic
system. In successive columns: (1) the isotopic reaction
iO+jOM
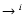 OjOM; (2) the reduced mass of the
reactants for M=5; (3) the reduced mass ratio
OjOM; (2) the reduced mass of the
reactants for M=5; (3) the reduced mass ratio
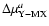 normalized to the reaction 16O+16OM for a=1;
(4) the product of the relative abundance with
normalized to the reaction 16O+16OM for a=1;
(4) the product of the relative abundance with
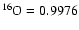 ,
,
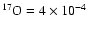 and
and
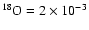 ;
(5) the
product of
;
(5) the
product of
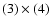 .
The 17O/16O ratio (
x'17/x'16) of the OOM molecule (noted
[17O/16O]
.
The 17O/16O ratio (
x'17/x'16) of the OOM molecule (noted
[17O/16O]
 is calculated from the values of the column
(5); that is:
is calculated from the values of the column
(5); that is:
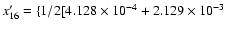
![$+ 4.070 \times 10^{-4 }+ 2.073 \times 10^{-3}] + 9.952 \times 10^{-1}\}$](/articles/aa/full/2004/09/aa4131/img9.gif) .
Similarly for
x'17 and x'18. This gives:
[17O/16O]
.
Similarly for
x'17 and x'18. This gives:
[17O/16O]
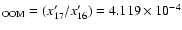 and [18O/16O]
and [18O/16O]
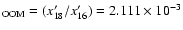 corresponding to
corresponding to
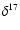 O = ([
O = ([
![$4.119 \times10^{-4 }/ (4 \times10^{-4}/0.9976)]-1)\times1000 = +27.3\mbox{\textperthousand}$](/articles/aa/full/2004/09/aa4131/img54.gif) and similarly
and similarly
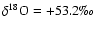 .
The
slope in the diagram
.
The
slope in the diagram
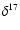 O versus
O versus
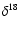 O is equal to
0.513.
O is equal to
0.513.
- Table 2:
A numerical example showing the non-mass-dependent
relations between the different reaction rates in the oxygen isotopic
system. In successive columns: (1) the isotopic reaction
iO+jOM
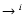 OjOM; (2) the reduced mass
ratio
OjOM; (2) the reduced mass
ratio
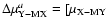 /
/
![$\mu _{\rm X-MX} ]^{a}$](/articles/aa/full/2004/09/aa4131/img17.gif) for a=0.1 and M=5; (3)
for a=0.1 and M=5; (3)
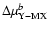 for b=
0.2; (4)
for b=
0.2; (4)
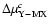 for c= 0.1; (5) the
reaction rate ratio R. Two situations are distinguished: (1) for
iO+ iOM (
for c= 0.1; (5) the
reaction rate ratio R. Two situations are distinguished: (1) for
iO+ iOM (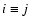 ),
),
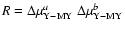 and for (2) jO+ iOM (
and for (2) jO+ iOM (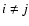 ),
),
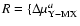 1/2
[
1/2
[
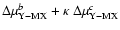 ]; calculations
are performed for
]; calculations
are performed for
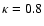 (6) The product of the
relative abundance with
(6) The product of the
relative abundance with
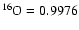 ,
,
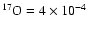 and
and
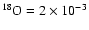 (7) the product of
(7) the product of
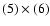 .
The 17O/16O ratio of the OOM molecule (noted
[17O/16O]
.
The 17O/16O ratio of the OOM molecule (noted
[17O/16O]
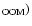 is calculated from the values of the column
[7]; that is for the abundance of 16O: {1/2 [
is calculated from the values of the column
[7]; that is for the abundance of 16O: {1/2 [
![$3.585 \times 10^{-4} + 1.789 \times 10^{-3} + 3.587\times 10^{-4 }+ 1.792 \times 10^{-3}] + 9.952 \times 10^{-1}$](/articles/aa/full/2004/09/aa4131/img27.gif) } and similarly for 17O and 18O. The
} and similarly for 17O and 18O. The
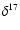 O (
O (
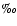 )
= ([17O/16O]
)
= ([17O/16O]
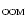 /[0.0004/0.9976])- 1)
/[0.0004/0.9976])- 1)
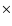 1000; similarly for
1000; similarly for
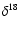 O (
O (
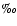 ). That is:
). That is:
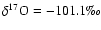 and
and
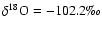 with
with
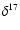 O/
O/
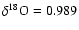 .
.