The results of a submillimetre wavelength spectral line survey between 455.1-507.4 GHz of the Orion-KL hot cloud core are reported. A total of 254 lines were detected to a main beam brightness temperature sensitivity
Glenn J. White1,2,3 - M. Araki4 - J. S. Greaves5 - M. Ohishi 6 - N. S. Higginbottom7
1 - Centre for Astrophysics & Planetary Science, University of Kent, Canterbury, Kent
CT2 7NR, UK
2 - Stockholm Observatory, 133 36 Saltsjöbaden, Sweden
3 - Astrophysics Group, The Cavendish Laboratory, University of Cambridge, Madingley Road, Cambridge CB3 OHE, UK
4 - Institute for Physical Chemistry, University of Basel, Klingelbergstrasse 80, 4056 Basel, Switzerland
5 - Royal Observatory, Blackford Hill, Edinburgh EH9 3HJ, UK
6 - National Astronomical Observatory of Japan, 2-21-1, Osawa, Mitaka, Tokyo 181-8588, Japan
7 - Department of Physics, Queen Mary & Westfield College, University of London, Mile End Road, London E1 4NS, UK
Received 11 June 2002 / Accepted 22 May 2003
Abstract
The results of a submillimetre wavelength spectral line survey between 455.1-507.4 GHz of the Orion-KL hot cloud core are reported. A total of 254 lines were detected to a main beam brightness temperature sensitivity
![]()
![]() 1-3 K. The detected lines are identified as being associated with 30 different molecular species or their isotopomeric variants. The strongest line detected was the J = 4-3 transition of the CO molecule. Apart from abundant diatomic rotors such as CO and CS, the spectrum is dominated by SO, SO
1-3 K. The detected lines are identified as being associated with 30 different molecular species or their isotopomeric variants. The strongest line detected was the J = 4-3 transition of the CO molecule. Apart from abundant diatomic rotors such as CO and CS, the spectrum is dominated by SO, SO![]() and CH
and CH![]() OH and large organic molecules such as (CH3)2O, CH
OH and large organic molecules such as (CH3)2O, CH![]() CN, C
CN, C![]() H
H![]() CN, C
CN, C![]() H
H![]() CN and HCOOCH
CN and HCOOCH![]() which make up
which make up ![]() 72% of the total number of lines; unidentified lines
72% of the total number of lines; unidentified lines ![]() 13%; and other lines the remaining
13%; and other lines the remaining ![]() 15
15![]() of the total. Rotational temperatures and column densities derived using standard rotation diagram analysis techniques were found to range from 70-300 K, and 1014-10
of the total. Rotational temperatures and column densities derived using standard rotation diagram analysis techniques were found to range from 70-300 K, and 1014-10
![]() cm
cm![]() respectively.
respectively.
Key words: molecules - star formation - molecular cloud
The chemistry of the Orion-KL molecular cloud core has been better studied than that of any other massive star formation region in the Galaxy (high spectral resolution spectroscopic surveys have been carried out by a number of authors including: 72-91 GHz Johansson et al.1984, 70-115 GHz Turner 1989,
138-151 GHz Lee et al.2001, 150-160 GHz Ziurys & McGonagle 1993, 215-247 GHz Sutton et al.1985, 216-242 GHz Blake et al.1986, 247-263 GHz Blake et al.1987, 257-273 GHz Greaves & White 1991, 330-360 GHz Jewell et al.1989, 325-360 GHz Schilke et al.1997, 342-359 GHz White et al.1986,
334-343 GHz Sutton et al.1995, 607-725 GHz Schilke et al.2001, 780-900 GHz Comito et al.2003, 190-900 GHz Serabyn & Weisstein 1995). Spectral line surveys can provide an unbiased view of the molecular constituents of the gas in star forming regions, and may be used to estimate the physical and chemical environment. We report here the first high spectral resolution survey in the 600 and 650 ![]() m atmospheric windows between frequencies of 455 and 507 GHz.
m atmospheric windows between frequencies of 455 and 507 GHz.
The spectral line survey was made using the James Clerk Maxwell telescope in Hawaii during October 1993 over the frequency range 455.1-507.4 GHz. This survey extended across
most of the two atmospheric transmission windows near 650 and 600 ![]() m. These windows are bracketed by strong telluric H2O absorption lines, and their transparency is highly dependent on the line of sight water vapour column. The data were collected using the JCMT facility receiver, RxC, operated in double-sideband mode. The adopted "on-source'' position was that of the "hot core'' close to IRc2 (
m. These windows are bracketed by strong telluric H2O absorption lines, and their transparency is highly dependent on the line of sight water vapour column. The data were collected using the JCMT facility receiver, RxC, operated in double-sideband mode. The adopted "on-source'' position was that of the "hot core'' close to IRc2 (![]() ,
, ![]() )
)
![]() ,
,
![]() 24
24![]() 23
23
![]() .
The pointing accuracy was measured to be good to better than 2
.
The pointing accuracy was measured to be good to better than 2
![]() rms, from observations of planets and compact calibrator sources used as standards at the telescope.
rms, from observations of planets and compact calibrator sources used as standards at the telescope.
The half power beam width and main beam efficiencies of the telescope were measured from observations of Mars, Jupiter and Uranus. These ranged from 11
![]() and 0.53 at the low frequency end of the spectral region to 10
and 0.53 at the low frequency end of the spectral region to 10
![]() and 0.49 at the upper end of the band. The receiver double sideband system noise temperatures were typically 1000-3000 K. The IF frequency was 3.94 GHz, and the spectra were processed using the JCMT facility 512 MHz bandwidth acousto-optical spectrometer, giving an effective spectral resolution of
and 0.49 at the upper end of the band. The receiver double sideband system noise temperatures were typically 1000-3000 K. The IF frequency was 3.94 GHz, and the spectra were processed using the JCMT facility 512 MHz bandwidth acousto-optical spectrometer, giving an effective spectral resolution of
![]() 0.6 km s-1.The spectral region was covered by stepping the local oscillator in 100 MHz steps across the whole spectral region - so that any part of the spectrum was redundantly observed at least four times
(i.e. at least twice in each sideband - and in many cases more times). Each
observation consisted of a 4-10 min integration (total on and off source), which was carried out in a "position-switched'' mode, where the telescope was alternated between the on-source position, and a "reference position'' located 2100
0.6 km s-1.The spectral region was covered by stepping the local oscillator in 100 MHz steps across the whole spectral region - so that any part of the spectrum was redundantly observed at least four times
(i.e. at least twice in each sideband - and in many cases more times). Each
observation consisted of a 4-10 min integration (total on and off source), which was carried out in a "position-switched'' mode, where the telescope was alternated between the on-source position, and a "reference position'' located 2100
![]() to the north. Previous observations of this "reference position'' have shown it to be free of significant molecular emission intense enough to affect the accuracy of the survey. The reference position was checked by position switching the telescope against several other positions that were located more than 10 degrees away from the Galactic Plane - and not known to be associated with the locations of any molecular clouds or enhanced interstellar extinction. The spectra were calibrated channel by channel using the standard JCMT three temperature chopper calibration scheme (hot and cold loads and the atmosphere). Observations of the sideband gains were measured by observations of spectral lines that were present in both sidebands. The main beam brightness temperature noise levels varied from 1-4 K in a 2 MHz channel (
to the north. Previous observations of this "reference position'' have shown it to be free of significant molecular emission intense enough to affect the accuracy of the survey. The reference position was checked by position switching the telescope against several other positions that were located more than 10 degrees away from the Galactic Plane - and not known to be associated with the locations of any molecular clouds or enhanced interstellar extinction. The spectra were calibrated channel by channel using the standard JCMT three temperature chopper calibration scheme (hot and cold loads and the atmosphere). Observations of the sideband gains were measured by observations of spectral lines that were present in both sidebands. The main beam brightness temperature noise levels varied from 1-4 K in a 2 MHz channel (![]() 1.3 km s-1).
1.3 km s-1).
During the data reduction, we attempted to recover an estimate of the single-sideband
spectrum, using data collected with a double-sideband receiver. A difficulty
common to sideband-deconvolution techniques is the uniqueness
of the deconvolution given the observational factors such as
pointing reproducibility, sideband gain imbalances, variable
calibration solutions as sky conditions change and contamination or blending with strong lines (of both terrestrial and extra-terrestrial origin) in the opposite sideband. A deconvolution technique was used that separated out the emission into the individual sidebands. The basis of this technique (which has been widely used by many observers at the JCMT and forms part of the facility software - although this paper gives the first description of the algorithm that is used) is to set up a series of linked equations for each channel in the DSB spectrum. The first
equation simply describes that the DSB line temperature is the sum of two intensities, one from the upper and one from the lower sideband (![]() and
and ![]() ,
say). The second equation refers to the same spectral channel but with a shift in the local oscillator setting by
,
say). The second equation refers to the same spectral channel but with a shift in the local oscillator setting by
![]() :
the DSB signal is then the sum of
:
the DSB signal is then the sum of ![]() and
and
![]() ,
if we consider the upper sideband to be the frame of reference and the shift to be positive (increased frequency). Similarly we can consider the lower sideband to be the frame of reference, and obtain a third DSB signal that is the sum of
,
if we consider the upper sideband to be the frame of reference and the shift to be positive (increased frequency). Similarly we can consider the lower sideband to be the frame of reference, and obtain a third DSB signal that is the sum of
![]() .
This equation set can be extended as far as desired by taking any of the line frequencies offset by
.
This equation set can be extended as far as desired by taking any of the line frequencies offset by
![]() and establishing which upper and lower sideband frequencies contribute to the observed DSB signal. The result is always to establish a set of n equations with (n + 1) variables that are linked to a particular channel of the DSB spectrum. A solution can only be found where one of the T(DSB) is consistent with zero within the noise level of the observations. Since this implies (if there are no absorption lines) that both
and establishing which upper and lower sideband frequencies contribute to the observed DSB signal. The result is always to establish a set of n equations with (n + 1) variables that are linked to a particular channel of the DSB spectrum. A solution can only be found where one of the T(DSB) is consistent with zero within the noise level of the observations. Since this implies (if there are no absorption lines) that both ![]() and
and ![]() are also zero within the noise, the equation set reduces to (n- 1) unknowns and two known values - hence all n equations can be solved. In the present survey, the local oscillator was stepped in 100 MHz intervals, and the number of equations used per spectral channel was typically 4-5. For several spectrally crowded regions, additional spectra were taken with different local oscillator offsets to improve the reliability of the deconvolution.
are also zero within the noise, the equation set reduces to (n- 1) unknowns and two known values - hence all n equations can be solved. In the present survey, the local oscillator was stepped in 100 MHz intervals, and the number of equations used per spectral channel was typically 4-5. For several spectrally crowded regions, additional spectra were taken with different local oscillator offsets to improve the reliability of the deconvolution.
Multiple coverage of individual parts of the spectrum provided sufficient redundancy to allow
single sideband spectra to be reconstructed. achieving an acceptable solution at most frequencies. The veracity of the technique could also be checked as lines from the lower and upper sidebands move in opposite directions in the DSB spectrum as the local oscillator frequency is stepped (in 100 MHz intervals). The deconvolution technique worked acceptably for more than 98% of the
whole spectral range covered, but the remaining ![]() 2% could not be solved because there was no signal consistent with zero in the DSB signal-set. A greater number of solutions could be found by extending the number of linked equations, but since the solutions are already of the form of sums and differences of DSB signals, the noise level will be increased if more differencing is involved. It is also inherent in the technique that there are a choice of solutions (for n equations and (n - 1) unknowns), so we have adopted the minimum SSB results and an initial DSB signal
2% could not be solved because there was no signal consistent with zero in the DSB signal-set. A greater number of solutions could be found by extending the number of linked equations, but since the solutions are already of the form of sums and differences of DSB signals, the noise level will be increased if more differencing is involved. It is also inherent in the technique that there are a choice of solutions (for n equations and (n - 1) unknowns), so we have adopted the minimum SSB results and an initial DSB signal ![]() 2
2![]() for the "consistent with zero''
DSB criterion. This minimises the level of spikes and should ensure
that temperature solutions are underestimated by less than
for the "consistent with zero''
DSB criterion. This minimises the level of spikes and should ensure
that temperature solutions are underestimated by less than ![]() 3
3![]() .
The locations of the parts of the spectrum for which we did not achieve a good deconvolution, are shown as horizontal bars under the spectrum in
Figs. 3-6. We also visually inspected at the locations in a spectrum where bright lines from the opposite sideband might have left small residual artifacts (sometimes known as "ghost features'' - Schilke et al.1997), as well as inspecting the emission in the opposite sideband to the locations of all of the "U-lines''.
.
The locations of the parts of the spectrum for which we did not achieve a good deconvolution, are shown as horizontal bars under the spectrum in
Figs. 3-6. We also visually inspected at the locations in a spectrum where bright lines from the opposite sideband might have left small residual artifacts (sometimes known as "ghost features'' - Schilke et al.1997), as well as inspecting the emission in the opposite sideband to the locations of all of the "U-lines''.
The intention of this paper is to present the data and some basic results. The spectrum shown
in Fig. 1 is crowded with many blended lines, and in many
places is confused - reflecting the rich and complex chemistry. A total of 254 lines were identified,
although there may be more lines than this blended together. The lines were clearly identified
using the JPL Sub-millimetre spectral line catalogues of Pickett et al.(1995), Pearson et al.(1996, 2000), Müller
![]() (2000) and other lists of line frequencies referred to later in this paper. A total of 98 lines (32% of
the total) could not be associated with known molecular transitions and have been designated as U-lines.
Although it is possible that some fraction of these may be artifacts of the deconvolution process - the fact that they are distributed throughout the survey, and do not tend to congregate in areas where the
deconvolution process was not successful, it is likely that many of these are real. Table 1 gives a breakdown of the number of transitions observed from each of the known species.
(2000) and other lists of line frequencies referred to later in this paper. A total of 98 lines (32% of
the total) could not be associated with known molecular transitions and have been designated as U-lines.
Although it is possible that some fraction of these may be artifacts of the deconvolution process - the fact that they are distributed throughout the survey, and do not tend to congregate in areas where the
deconvolution process was not successful, it is likely that many of these are real. Table 1 gives a breakdown of the number of transitions observed from each of the known species.
| Molecule |
|
Error |
|
Error | Number | Note |
| cm-3 | cm-3 | K | K | of lines | ||
| (CH3)2O |
|
|
157 | 30 | 27(26) lines | |
| C2H3CN |
|
|
180 | 47 | 13(6) lines | [1] |
| C2H5CN |
|
|
150 | 12 | 27(23) lines |
|
|
|
|
239 | 12 | 27(23) lines |
|
|
| C2H5OH |
|
|
70 | - | 8(6) lines |
|
|
|
|
264 | 196 | 8(6) lines | ||
| CH2NH |
|
|
150 | - | 3(2) lines |
|
| CH3CN |
|
|
227 | 21 | 19 lines | |
| CH313CN |
|
|
227 | - | 2 lines |
|
|
|
- | 74 | - | 2 lines | ||
| CH3OH |
|
|
599 | 295 | 24(23) lines | |
| H2CO |
|
- | 166 | - | 2(1) lines |
|
| H213CO |
|
|
166 | - | 2 lines |
|
| HC3N |
|
- | 164 | - | 2 lines | |
| HCOOCH3 |
|
|
301 | 95 | 26(24) lines | |
| HNCO |
|
|
150 | 14 | 4(3) lines | |
| NH2CN |
|
|
200 | - | 3 lines |
|
|
|
|
100 | - | 3 lines |
|
|
| OCS |
|
- | 106 | - | 2 lines | |
| SO |
|
- | 72 | - | 2(1) lines |
|
| 34SO |
|
|
89 | 43 | 5(4) lines | |
| SO2 |
|
|
136 | 9 | 35(28) lines | |
| 34SO2 |
|
- | 156 | - | 3(2) lines | |
| 13CS |
|
- | 120 | - | 1 line |
|
| 30SiO |
|
- | 50 | - | 1 line |
|
| CH3CHO |
|
- | 81 | - | 1 line |
|
| CI |
|
- | 30 | - | 1 line |
|
| CO |
|
- | 200 | - | 4 lines (1 transition) |
|
| DCN |
|
- | 200 | - | 1 line |
|
| HCOOH |
|
- | 100 | - | 1 line |
|
| HDO |
|
- | 164 | - | 1 line |
|
| N2O |
|
- | 230 | - | 1 line |
|
| NH2CHO |
|
- | 81 | - | 1 lines |
|
| NH2D |
|
- | 160 | - | 1 line |
|
|
LTE rotation temperatures and beam averaged
|
These data were used
to estimate the rotational temperature,
![]() ,
and column densities,
,
and column densities,
![]() ,
of the various
species using the relationship given in Eq. (1).
,
of the various
species using the relationship given in Eq. (1).
One of the objectives in analysing spectral line survey data is to determine molecular parameters, such as rotation temperatures, column densities etc. The approach commonly adopted has been to use a "rotation diagram'' to estimate these parameters. In this, an optically thin transition produces an antenna temperature that is proportional to the column density in the upper level of the transition being observed. If all transitions are thermalised and the kinetic temperature is known, then a single integrated line intensity and be used to estimate the total column density of the species in question. The rotational temperature diagram is a plot showing the column density per statistical weight of a number of molecular energy levels, as a function of energy above the ground state - in local thermodynamic equilibrium (LTE), this is equivalent to a Boltzmann distribution. A plot of the natural logarithm of the column density, N, divided by the degeneracy g, versus the Energy E of the final state of the level (expressed in units of degrees K) E/k, will lead to a straight line fit with a slope of 1/T, where g is the statistical weight of level u lying at E energy above the ground state, and T is the rotational temperature. This is equivalent to the kinetic temperature in the limit where all of the levels are thermalised.
One problem for the rotation diagram method is that it may underestimate the total column density if
some of the lines fitted are optically thick, or LTE conditions do not hold, or if the background radiation is non-negligible (Turner 1991; Goldsmith & Langer 1999; Nummelin et al.2000; Schilke et al.2001).
This can be however be addressed by using less abundant isotopomeric variants that allow estimates to be made of the optical depths, and then using these to correct the column density estimates. It has however been widely used in past molecular line survey studies, because of its computational simplicity, and the absence of a need to have observations of an isotopomer. In this paper we give results from the traditional rotation-diagram technique (Table 1), except in cases pointed out in the Table and text where the results may be affected by high optical depth effects. We have examined a number of cases (CH![]() CN, SO
CN, SO![]() ,
OCS, SO, CH
,
OCS, SO, CH![]() OH and
OH and ![]() SO in some detail - see for example Sects. 4.3 and 4.2 - calculating the optically thick and thin total column densities - and found the use of the rotational temperature technique to provide similar column density estimates are quite similar to the rotational diagram values (see for example discussion of opacities in Sect. 4.2). Another indicator as to whether opacity corrections are important, is to look at the rotational temperature diagrams (see Fig. 7), to see whether there are data points that deviate noticeably from a straight line fit - and which may indicate that the line has saturated. Lee et al. (2001) applied this kind of constraint to seven molecules (CH
SO in some detail - see for example Sects. 4.3 and 4.2 - calculating the optically thick and thin total column densities - and found the use of the rotational temperature technique to provide similar column density estimates are quite similar to the rotational diagram values (see for example discussion of opacities in Sect. 4.2). Another indicator as to whether opacity corrections are important, is to look at the rotational temperature diagrams (see Fig. 7), to see whether there are data points that deviate noticeably from a straight line fit - and which may indicate that the line has saturated. Lee et al. (2001) applied this kind of constraint to seven molecules (CH![]() OH, HCOOCH
OH, HCOOCH![]() ,
CH
,
CH![]() OCH
OCH![]() ,
C
,
C![]() H
H![]() CN, SO
CN, SO![]() ,
CH
,
CH![]() CN, and H
CN, and H![]() CO), finding that the majority could to first order be treated using the rotational technique. The issue of opacity will be further discussed in the relevant sections dealing with molecules that may be opaque - where we also conclude that the effects of opacity are minor in the analysis of data from this survey, and that our justification of using the optically thin rotational temperature technique is adequate although there clear examples (Schilke et al.1997, 2001) where opacity corrections are required for CH
CO), finding that the majority could to first order be treated using the rotational technique. The issue of opacity will be further discussed in the relevant sections dealing with molecules that may be opaque - where we also conclude that the effects of opacity are minor in the analysis of data from this survey, and that our justification of using the optically thin rotational temperature technique is adequate although there clear examples (Schilke et al.1997, 2001) where opacity corrections are required for CH![]() OH.
OH.
Full details of the detected lines are given in Tables 2-6.
Individual spectra are shown in more detail in Figs. 3, 4,
5 and 6, along with identifications of the prominent lines. Previous studies (Blake et al. 1987; Schilke et al.1997, 2001) have shown that there are four characteristic velocity components in Orion spectra: the extended ridge
- which is ambient gas in the Orion Molecular cloud (
![]()
![]() 9 km s-1
9 km s-1
![]() km s-1), the compact ridge - which is a compact clump lying about 10
km s-1), the compact ridge - which is a compact clump lying about 10
![]() south-west of the hot core (
south-west of the hot core (
![]()
![]() 8 km s-1
8 km s-1
![]() 3 km s-1), the plateau - which which has been identified as the outflow, and is associated with the broadest spectral lines (
3 km s-1), the plateau - which which has been identified as the outflow, and is associated with the broadest spectral lines (
![]()
![]() 6-10 km s-1
6-10 km s-1
![]() 20 km s-1), and the hot core that lies close to the infrared source IRc2 (
20 km s-1), and the hot core that lies close to the infrared source IRc2 (
![]()
![]() 3-6 km s-1
3-6 km s-1
![]() 5-10 km s-1). Most of the observed lines show velocities within these ranges, except a few lines which may suffer from blending (e.g. lines at 455.7798, 459.7069, 466.7817, 495.0007, 496.44095, 503.8517, 504.7281 GHz). The superposed dotted line shows the normalised atmospheric absorption (see Fig. 1 caption)
under conditions typical for the survey.
5-10 km s-1). Most of the observed lines show velocities within these ranges, except a few lines which may suffer from blending (e.g. lines at 455.7798, 459.7069, 466.7817, 495.0007, 496.44095, 503.8517, 504.7281 GHz). The superposed dotted line shows the normalised atmospheric absorption (see Fig. 1 caption)
under conditions typical for the survey.
| Frequency | Species | Transition | Peak | Width | Vel | Notes |
| MHz |
|
km s-1 | km s-1 | |||
| 455.160796 | SO2 | 215,17-214,18 | 23.2 | 33.3 | 8.2 | |
| 455.250265 | SO2 | 295,25-294,26 | 14.8 | 19.6 | 6.9 | |
| 455.353721 | SO2 | 185,13-184,14 | 17.6 | 8.0 | 5.4 | |
| 455.450207 | U-line | 4.3 | 9.4 | |||
| 455.617930 | CH3OH | 61-50 | 36.1 | 10.6 | 9.1 | |
| 455.779855 | SO2 | 113,9-102,8 | 60.2 | 13.8 | 1.6 | Blend with C2H3CN |
| 456.271440 | CH3OH | 181-172 | 20.2 | 6.2 | 8.4 | |
| 456.359253 | SO2 | 195,15-194,16 | 29.5 | 11.6 | 4.4 | |
| 456.513228 | U-line | 18.8 | 4.3 | |||
| 456.553270 | U-line | 4.7 | 16.6 | C3H2 220,22-210,21 ? | ||
| 456.800826 | CH3OH | 18-2-18-1 | 12.7 | 7.9 | 5.3 | Blend with C2H5CN |
| 456.936702 | (CH3)2O | 153,12-142,13 | 5.0 | 9.3 | 6.9 | 4 lines blended |
| 457.006904 | CH3OH | 112-111 | 23.7 | 7.6 | 8.2 | |
| 457.248931 | CH3OH | 19-1-18-2 | 9.3 | 15.6 | 9.9 | |
| 457.325926 | SO2 | 165,11-164,12 | 30.4 | 11.3 | 2.2 | Blend with C2H3CN |
| 457.381156 | SO2 | 158,8-167,9+158,7-167,10 | 7.5 | 14.6 | 7.8 | |
| 457.472642 | SO2 | 175,13-174,14 | 50.1 | 11.4 | 5.2 | |
| 457.584559 | U-line | 7.0 | 3.5 | |||
| 457.637840 | U-line | 3.5 | 7.3 | |||
| 457.723839 | C2H5CN | 518,44-508,43 | 5.1 | 5.2 | 11.5 | |
| 457.736126 | HCOOCH3 | 403,37-393,36 | 9.2 | 6.0 | 7.4 | |
| 457.783725 | U-line | 13.3 | 9.4 | |||
| 457.822031 | U-line | 9.1 | 28.5 | |||
| 457.839618 | U-line | 4.7 | 4.3 | SO2 v2 = 1 ? | ||
| 457.878236 | C2H5CN | 532,52-522,51 | 5.4 | 4.3 | 5.2 | |
| 457.890562 | C2H5CN | 285,23-274,24 | 5.9 | 6.0 | 9.3 | |
| 457.947140 | HCOOCH3 | 386,32-375,32 | 3.4 | 6.0 | 10.5 | |
| 457.985674 | HCOOH | 203,17-193,16 | 3.8 | 2.8 | 9.6 | |
| 458.022659 | C2H5CN | 484,45-473,44 | 4.9 | 10.3 | 7.2 | |
| 458.148139 | CH3OH | 101-92 | 15.3 | 7.0 | 7.0 | |
| 458.276916 | (CH3)2O | 251,24-242,23 | 11.6 | 3.9 | 8.8 | 4 lines blended |
| 458.389953 | SO2 | 155,11-154,12 | 40.8 | 25.2 | 6.9 | |
| 458.433457 | C2H5OH | 281,28- 271,27 | 8.2 | 5.3 | 6.1 | |
| 458.512326 | HCOOCH3 | 41 2/3/2/2,39-40 3/3/2/2,38 | 20.7 | 12.2 | 8.5 | 4 lines blended |
| 458.536360 | SO2 | 145,9-144,10 | 34.8 | 16.7 | 5.9 | |
| 458.601426 | HCOOCH3 | 3611,25-3511,24 | 7.1 | 8.0 | 9.9 | |
| 458.619675 | SO2 | 315,27-314,28 | 8.6 | 9.8 | 3.8 | |
| 458.998198 | CH3CN | J=25-24 K = 9 | 3.9 | 5.7 | 7.3 | |
| 459.054419 | U-line | 20.5 | 22.1 | |||
| 459.076694 | SO2 | 135,9-134,10 | 36.0 | 14.1 | 5.0 | |
| 459.150830 | CH3CN | J = 25-24 K = 8 | 4.3 | 11.2 | 4.9 | |
| 459.257959 | U-line | 23.3 | 24.2 | |||
| 459.378018 | U-line | 8.3 | 6.1 | |||
| 459.395715 | CH3CN | J=25-24 K = 6 | 17.7 | 10.4 | 5.4 | Blend with CH313CN J=25-24 K =3 |
| 459.443401 | CH313CN | J=25-24 K = 2 | 4.7 | 13.9 | 9.6 | |
| 459.492742 | CH3CN | J=25-24 K =5 | 20.4 | 16.7 | 5.3 | Blend with CH313CN J=25-24 K = 0,1 |
| 459.511802 | NH2CN | 23 4,20/19-22 4,19/18 | 16.0 | 9.3 | 9.0 | |
| 459.534263 | SO2 | 115,7-114,8 | 39.4 | 19.7 | 5.2 | |
| 459.569872 | CH3CN | J=25-24 K =4 | 17.3 | 13.6 | 6.6 | |
| 459.631405 | CH3CN | J=25-24 K =3 | 30.9 | 13.0 | 6.6 | |
| 459.678274 | CH3CN | J=25-24 K =2 | 37.8 | 10.8 | 4.8 | |
| 459.706888 | CH3CN | J=25-24 K =0,1 | 35.4 | 15.5 | 3.3 | 2 lines blended |
| 459.760261 | HNCO | 211,21-201,20 | 14.8 | 7.2 | 6.1 | |
| 459.801217 | SO2 | 95,4-94,5+95,5-94,6 | 35.0 | 22.8 | 8.1 | |
| 459.881164 | SO2 | 85,3-84,4+85,4-84,5 | 49.9 | 32.7 | 7.6 | |
| 459.942528 | SO2 | 75,3-74,4 | 57.9 | 26.2 | 4.8 | |
| 459.983548 | SO2 | 55,0-54,1+55,1-54,2 | 53.6 | 25.6 | 11.4 |
| Frequency | Species | Transition | Peak | Width | Vel | Notes |
| MHz |
|
km s-1 | km s-1 | |||
| 460.069658 | C2H5CN | 314,27-303,28 | 2.6 | 23.5 | 7.3 | |
| 460.214973 | HCOOCH3 | 376,31-366,30 | 6.5 | 3.6 | 8.8 | |
| 460.272941 | 34SO2 | 64,2-53,3 | 7.5 | 31.1 | 7.8 | |
| 460.281266 | HCOOCH3 | 431,43-421,42 | 8.8 | 6.9 | 9.6 | |
| 460.306531 | C2H5CN | 2215,7/8-23 14,10/9 | 4.0 | 2.3 | 7.1 | |
| 460.387575 | HCOOCH3 | 3712,26-3612,25 | 3.1 | 27.3 | 8.0 | 2 lines blended |
| 460.466108 | U-line | 3.0 | 13.7 | |||
| 460.545686 | U-line | 6.2 | 6.2 | |||
| 460.591671 | U-line | 5.0 | 3.9 | |||
| 460.878573 | CH3OH | 92-81 | 24.4 | 6.6 | 7.3 | |
| 460.913504 | U-line | 3.8 | 5.0 | |||
| 461.040768 | CO | J = 4-3 | 182.0 | 51.0 | 9.0 | |
| 461.342825 | U-line | 5.0 | 7.5 | |||
| 461.373909 | HNCO | 212,19-202,18 | 7.4 | 6.1 | 5.7 | |
| 461.709888 | HCOOCH3 | 307,24-296,23 | 5.5 | 8.4 | 9.6 | |
| 461.756285 | CH3OH | 150-141 | 43.8 | 11.8 | 7.8 | |
| 461.880198 | SO2 | 209,11-218,14+209,12-218,13 | 7.9 | 12.6 | 6.2 | |
| 461.910483 | OCS | J=38-37 | 18.1 | 11.8 | 7.2 | |
| 462.035190 | CH3OH
|
106-115+ | 8.1 | 13.0 | 9.8 | |
| 462.036358 | C2H5OH | 135,9-124,8 | 8.1 | 13.0 | 12.5 | Blended with CH3NC |
| 462.138660 | HCOOCH3 | 3711,27-3611,26 | 3.5 | 7.0 | 8.4 | |
| 462.236037 | 34SO | 42.0 | 19.3 | |||
| 462.334032 | 13CS | J=10-9 | 14.6 | 16.4 | 6.8 | Blended with C2H5CN |
| 463.017334 | SO2 | 122,10-111,11 | 21.3 | 23.0 | 5.2 | |
| 463.122260 | HNCO | 211,20-201,19 | 8.5 | 6.8 | 7.2 | |
| 463.329490 | SO2 | 354,32-353,33 | 5.6 | 14.3 | 6.9 | |
| 463.720861 | HC3N | J=51-50 | 8.2 | 13.2 | 5.4 | |
| 463.836119 | 34SO2 | 260,26-251,25 | 8.2 | 10.4 | 9.1 | |
| 464.026804 | U-line | 6.8 | 25.2 | |||
| 464.091929 | 34SO | NJ=1111-1010 | 20.8 | 25.8 | 11.6 | |
| 464.200231 | CH3CHO | 74,4-63,3 | 4.2 | 5.8 | 7.9 | Blend with HCOOCH3 |
| 464.200231 | HCOOCH3 | 3710,28-3610,27 | 4.2 | 5.8 | 9.5 | Blend with CH3CHO |
| 464.293855 | SO2 | 335,29-334,30 | 4.1 | 10.8 | 5.1 | |
| 464.570359 | CH2NH | 71,6-61,5 | 4.4 | 16.8 | 8.5 | |
| 464.730426 | U-line | 12.9 | 6.6 | |||
| 464.837513 | CH3OH | 92-91 | 34.0 | 11.3 | 7.2 | Blend with C2H5CN 92-91 |
| 464.928173 | HDO | 10,1-00,0 | 33.5 | 16.2 | 6.6 | |
| 465.061222 | C2H3CN | 522,49-492,48 v2 = 1 | 1.9 | 11.1 | 12.3 | |
| 465.078910 | U-line | 3.6 | 4.1 | |||
| 465.352350 | 34SO | NJ=1112-1011 | 30.5 | 23.0 | 7.2 | |
| 465.512820 | U-line | 2.4 | 5.4 | |||
| 465.543972 | (CH3)2O | 213,19-202,18 | 3.6 | 11.2 | 5.9 | 4 lines blended |
| 465.565977 | HCOOCH3 | 361,35-351,34 | 2.4 | 14.7 | 9.0 | |
| 465.605514 | C2H5CN | 52 12,41/40-51 12,40/39 | 4.0 | 11.2 | 4.4 | |
| 465.628191 | SO2 | 292,28-291,29 | 13.0 | 8.4 | 5.1 | |
| 465.713762 | C2H5CN | 515,46-505,45 | 7.4 | 13.2 | 5.9 | |
| 465.732611 | NH2CHO | 221,21-211,20 | 16.1 | 9.7 | 6.7 | |
| 465.758340 | SO2 | 260,26-251,25 | 45.3 | 15.8 | 4.4 | |
| 465.882288 | (CH3)2O | 144,11-133,10 | 4.0 | 9.9 | 6.1 | Blend with SO2 |
| 465.882288 | SO2 | 2510,16-269,17+2510,15-269,18 | 4.0 | 9.9 | 8.7 | Blended with (CH3)2O |
| 465.922779 | SO2 v2 = 1 | 185,13-184,14 | 2.2 | 9.5 | 7.9 | |
| 465.956339 | 30SiO | J=11-10 | 7.7 | 15.1 | 8.8 | |
| 466.781659 | C2H3CN | 49 13,36/37-48 13,35/36 v1 = 1 | 13.8 | 30.7 | 11.9 | Blended with C2H5CN |
| 466.781659 | C2H5CN | 528,44-518,43 | 13.8 | 30.7 | 3.8 | Blend with C2H3CN |
| 466.892775 | SO2 | 406,34-405,35 | 5.5 | 5.5 | 5.7 | |
| 466.999547 | U-line | 3.1 | 5.6 | |||
| 467.048535 | HCOOCH3 | 38 23,16/15-37 23,15/14 | 5.6 | 14.7 | 8.9 |
| Frequency | Species | Transition | Peak | Width | Vel | Notes |
| MHz |
|
km s-1 | km s-1 | |||
| 467.212513 | HCOOCH3 | 3822,17-3722,16 | 4.6 | 5.5 | 8.6 | |
| 467.282605 | C2H3CN | 49 16,33/34-48 16,32/33 v1 = 1 | 6.9 | 7.6 | 8.2 | |
| 467.318607 | HCOOCH3 | 396,34-386,33 | 3.9 | 19.2 | 9.4 | 2 lines blended |
| 467.475284 | U-line | 10.5 | 8.3 | |||
| 467.532613 | (CH3)2O | 260,26-251,25 | 6.2 | 5.4 | 7.7 | 4 lines blended |
| 467.586402 | HCOOCH3 | 395,34-385,33 | 3.7 | 10.5 | 8.3 | |
| 467.627790 | HCOOCH3 | 199,10-188,10 | 2.8 | 10.2 | 8.5 | |
| 467.658900 | U-line | 6.0 | 17.2 | |||
| 467.670464 | C2H5OH | 153,12-142,12 | 10.4 | 6.4 | 9.4 | |
| 467.715623 | (CH3)2O | 261,26-251,25 | 14.4 | 13.0 | 5.6 | 4 lines blended |
| 467.765733 | 13CH3OH | 101-91
|
7.1 | 6.4 | ||
| 468.048223 | C2H5CN | 550,55-540,54 | 2.9 | 12.8 | 6.5 | |
| 468.300479 | CH3OH | 82-81 | 20.3 | 8.4 | 4.7 | Blend with U-line |
| 468.345184 | U-line | 7.6 | 6.0 | |||
| 491.943519 | SO2 | 74,4-63,3 | 49.4 | 10.3 | 3.6 | |
| 491.978910 | H2CO | 71,7-61,6 | 45.0 | 13.6 | 2.6 | |
| 492.160626 | CI | 3P1-3P1 | 17.9 | 5.0 | 9.0 | |
| 492.281601 | CH3OH | 41-30 | 36.9 | 10.4 | 7.2 | |
| 492.470010 | U-line | 5.2 | 8.5 | |||
| 492.544658 | C2H5CN | 55 11,45/44-54 11,44/43 | 3.5 | 4.3 | 5.3 | |
| 492.695825 | 13CH3OH | 9.9 | 4.7 | 8.1 | ||
| 492.784191 | (CH3)2O | 96,4-85,4 | 20.7 | 8.4 | 8.6 | 8 lines blended |
| 493.260821 | C2H5CN | 581/0,58-571/0,57 | 4.4 | 7.4 | 6.2 | |
| 493.370372 | U-line | 9.6 | 11.4 | |||
| 493.701318 | CH3OH | 53-42 | 23.7 | 11.4 | 7.6 | |
| 493.735068 | CH3OH | 53-42 | 29.0 | 9.3 | 8.1 | |
| 494.460056 | NH2D | 11,0 - 00,0 | 10.5 | 8.6 | 5.8 | |
| 494.484560 | CH3OH | 72-71 | 50.8 | 10.3 | 7.2 | |
| 494.555486 | SO2 | 1327-1228 | 2.5 | 4.2 | 7.6 | |
| 494.557464 | C2H5CN | 557,49-547,48 | 2.5 | 4.2 | 5.4 | Blend with C2H5OH |
| 494.557464 | C2H5OH | 109,1-98,1 | 2.5 | 4.2 | 8.7 | Blend with C2H5CN |
| 494.575046 | C2H5CN | 325,27-314,28 | 2.6 | 6.4 | 9.5 | |
| 494.596465 | (CH3)2O | 289,19-288,20+289,20-288,21 | 5.7 | 5.3 | 7.7 | 8 lines blended |
| 494.756403 | C2H3CN | 52 17,35/36-51 17,34/35 v = 0 | 14.1 | 17.1 | 5.2 | |
| 494.781464 | SO2 | 123,9-112,10 | 45.0 | 26.6 | 7.9 | |
| 494.899521 | C2H5OH | 283,25-273,24 | 1.8 | 10.5 | 3.1 | |
| 495.000693 | (CH3)2O | 279,18-278,19+279,19-278,20 | 1.6 | 15.1 | 10.9 | 8 lines blended |
| 495.222806 | (CH3)2O | 271,26-262,25 | 7.8 | 14.4 | 7.5 | 4 lines blended |
| 495.486885 | HCOOCH3 | 397,32-387,31 | 12.5 | 4.8 | 7.4 | |
| 495.821871 | C2H5OH | 264,22-253,22 | 2.1 | 2.8 | 3.5 | |
| 495.842389 | SO2 | 138,6-147,7+138,5-147,8 | 9.8 | 20.5 | 8.5 | |
| 495.979430 | (CH3)2O | 249,15-248,16+249,16-248,17 | 8.7 | 5.8 | 7.1 | 8 lines blended with CH3CN |
| 495.979430 | CH3CN | J=27-26 K = 7 | 8.7 | 5.8 | 7.0 | Blend with (CH3)2O |
| 496.107704 | CH3CN | J=27-26 K = 6 | 11.5 | 11.6 | 4.6 | |
| 496.207453 | CH3CN | J=27-26 K = 5 | 8.8 | 13.3 | 6.6 | |
| 496.295793 | CH3CN | J=27-26 K = 4 | 13.3 | 11.1 | 4.8 | |
| 496.361787 | CH3CN | J=27-26 K = 3 | 19.2 | 11.6 | 5.1 | |
| 496.409463 | CH3CN | J=27-26 K = 2 | 20.7 | 12.5 | 5.0 | |
| 496.440950 | (CH3)2O | 249,15-248,16+249,16-248,17 | 26.6 | 17.0 | 10.9 | 8 lines blended with CH3CN |
| 495.175350 | CH3OH | 70-6-1 | 39.7 | 10.2 | 7.7 | |
| 496.440950 | CH3CN | J=27-26 K = 1 | 26.6 | 17.0 | 3.2 | 2 lines blended, Blend with (CH3)2O |
| 496.518825 | H213CO | 72,6-62,5 | 3.1 | 7.2 | 6.3 | |
| 496.633861 | (CH3)2O | 219,12-218,13+219,13-218,14 | 7.0 | 5.2 | 8.4 | 8 lines blended |
| 496.764492 | C2H3CN | 525,47-515,46 v2 = 1 | 2.2 | 14.4 | 14.1 | |
| 496.796267 | (CH3)2O | 209,11-208,12+209,12-208,13 | 5.8 | 3.8 | 8.4 | 8 lines blended |
| 496.928524 | CH3OH | 140-131 | 16.7 | 9.2 | 6.5 | |
| 497.051216 | (CH3)2O | 189,9-188,10+189,10-188,11 | 7.2 | 14.8 | 8.6 | 8 lines blended |
| Frequency | Species | Transition | Peak | Width | Vel | Notes |
| MHz |
|
km s-1 | km s-1 | |||
| 497.085708 | C2H5OH | 97,3/2-86,2/3 | 3.1 | 10.4 | 10.3 | |
| 497.119025 | H213CO | 74,4/3-64,3/2 | 4.8 | 6.9 | 6.6 | |
| 497.149132 | (CH3)2O | 179,8-178,9+179,9-178,10 | 6.4 | 7.3 | 8.5 | 8 lines blended |
| 497.226494 | (CH3)2O | 169,7-168,8+169,8-168,9 | 5.5 | 4.8 | 10.4 | 8 lines blended |
| 497.295820 | (CH3)2O | 159,6-158,7+159,7-158,8 | 5.2 | 3.2 | 8.1 | 4 lines blended |
| 497.364091 | U-line | 3.7 | 7.0 | |||
| 497.388286 | (CH3)2O | 139,4-138,5+139,5-138,6 | 8.5 | 10.8 | 8.4 | 8 lines blended |
| 497.404849 | U-line | 4.4 | 1.5 | |||
| 497.417697 | (CH3)2O | 129,3-128,4+129,4-128,5 | 9.4 | 8.4 | 9.4 | 8 lines blended |
| 497.442262 | (CH3)2O | 119,2-118,3+119,3-118,4 | 4.2 | 9.4 | 8.3 | 8 lines blended with HCOOCH3 |
| 497.442262 | HCOOCH3 | 4013,28-3913,27 +4015,26-3913,27 | 4.2 | 9.4 | 9.0 | 2 lines blended with (CH3)2O |
| 497.516476 | C2H3CN | 601,59-601,60 v = 0 | 4.8 | 6.1 | 6.8 | |
| 497.556527 | U-line | 1.5 | 9.4 | |||
| 497.606988 | (CH3)2O | 243,22-232,21 | 4.3 | 2.7 | 8.5 | 4 lines blended |
| 497.661575 | C2H5CN | 563,53-553,52 | 4.9 | 6.0 | 3.5 | Blend with CH3CN v = 8 |
| 497.661575 | CH3CN v8=1 | J=27-26 K=4 -l | 4.9 | 6.0 | 4.9 | Blend with C2H5CN |
| 497.734975 | CH3CN v8=1 | J=27-26 K=3 -l | 3.5 | 4.5 | 8.8 | |
| 497.828685 | CH3OH | 82-81 - | 14.0 | 9.4 | 8.7 | |
| 497.905738 | CH3CN v8=1 | J=27-26 K=4 +l | 3.6 | 6.4 | 5.9 | |
| 498.288253 | C2H5CN | 572,55-562,54 | 2.2 | 6.6 | 5.1 | |
| 498.327509 | OCS | J=41-40 | 12.6 | 10.2 | 6.6 | |
| 498.412959 | CH3CN v8=1 | J=27-26 K=1 +l | 6.4 | 6.8 | 6.9 | |
| 498.431868 | 34SO2 | 84,4-73,5 | 5.9 | 12.2 | 10.0 | |
| 498.596075 | HCOOCH3 | 417,35-407,34 | 2.2 | 8.1 | 9.2 | |
| 498.677131 | 13CH3OH | 82-81 | 8.5 | 7.3 | 4.8 | |
| 498.785157 | C2H5CN | 554,51-544,50 | 2.0 | 9.8 | 4.5 | |
| 498.849325 | (CH3)2O | 164,13-153,12 | 7.7 | 7.0 | 8.5 | 4 lines blended with HCOOCH3 |
| 498.849325 | HCOOCH3 | 425,37-415,36 | 7.7 | 7.0 | 6.8 | Blend with (CH3)2O |
| 498.984855 | C2H5CN | 556,49-546,48 | 5.4 | 23.7 | 12.0 | |
| 499.198900 | HCOOCH3 | 435,39-425,38 | 3.0 | 6.5 | 8.1 | |
| 499.283980 | C2H3CN | 204,17-193,16 v1 = 1 | 3.4 | 13.9 | 7.3 | Blend with C2H5CN |
| 499.283980 | C2H5CN | 178,10/9-167,9/10 | 3.4 | 13.9 | 3.4 | Blend with C2H3CN |
| 499.367702 | (CH3)2O | 272,26-261,25 | 3.9 | 6.1 | 7.1 | 4 lines blended |
| 499.892902 | C2H5CN | 581/2,57-571/2,56 | 9.1 | 8.0 | 5.4 | |
| 499.917085 | NH2CN | 252,23-242,22 | 8.1 | 8.1 | 8.2 | 13C34S J=11-10 ? |
| 499.944145 | U-line | 10.2 | 9.6 | |||
| 500.038140 | HC3N | J=55-54 | 9.0 | 7.9 | 6.8 | |
| 500.052075 | NH2CN | 253,22-243,21 | 8.0 | 11.8 | 4.9 | |
| 500.075688 | SO2 v2=1 | 74,4-63,3 | 3.8 | 11.5 | 8.6 | |
| 500.364813 | C2H3CN | 416,35-415,36 v2 = 1 | 4.9 | 5.7 | 6.8 | |
| 500.432652 | SO2 | 189,9-198,12+189,10-198,11 | 20.1 | 6.0 | 8.3 | |
| 500.457324 | C2H5CN | 129,3/4-118,4/3 | 22.3 | 8.2 | 2.9 | |
| 500.475691 | CH2NH | 22,1-21,2 | 18.0 | 7.5 | 6.3 | |
| 500.637890 | C2H5CN | 355,31-344,30 | 5.9 | 5.1 | 5.5 | |
| 500.661562 | SO2 | 312,30-311,31 | 9.0 | 13.3 | 5.3 | |
| 501.087663 | C2H3CN | 551,55-541,54 v1 = 1 | 11.7 | 12.8 | 10.4 | |
| 501.116812 | SO2 | 280,28-271,27 | 21.5 | 19.1 | 3.5 | |
| 501.592173 | CH3OH | 92-91 - | 32.9 | 11.7 | 7.1 | |
| 501.656920 | C2H5CN | 591/0,59-581/0,58 | 5.6 | 8.1 | 8.2 | |
| 501.679997 | CH2NH | 80,8-70,7 | 4.4 | 11.5 | 9.0 | |
| 501.891221 | (CH3)2O | 303,27-294,26 | 2.8 | 12.0 | 9.1 | 4 lines blended |
| 502.181627 | HCOOCH3 | 1512,3/4-1411,4/3 | 4.6 | 7.0 | 8.2 | |
| 502.303684 | N2O | J=20-19 | 3.4 | 17.4 | 4.7 | |
| 503.018652 | (CH3)2O | 280,28-271,27 | 10.4 | 10.7 | 7.4 | 4 lines blended |
| 503.107069 | (CH3)2O | 281,28-270,27 | 5.1 | 5.2 | 9.2 | 4 lines blended |
| 503.210868 | HCOOCH3 | 408,32-398,31 | 2.7 | 7.6 | 8.5 | |
| 503.518307 | HCOOCH3 | 229,13-218,14 | 6.2 | 6.1 | 7.6 |
| Frequency | Species | Transition | Peak | Width | Vel | Notes |
| MHz |
|
km s-1 | km s-1 | |||
| 503.574370 | C2H3CN | 53 14,39/40-52 14,38/39 v = 0 | 9.5 | 5.6 | 7.9 | Blend with CH3OH |
| 503.574370 | CH3OH | 76-85 | 9.5 | 5.6 | 7.5 | Blend with C2H3CN |
| 503.707705 | HCOOCH3 | 4126,16-4026,15 | 3.3 | 6.8 | 8.4 | |
| 503.836578 | C2H5CN | 286,23-275,22 | 3.4 | 2.1 | 6.8 | |
| 503.851690 | SO | NJ=1514-1414 | 8.3 | 10.9 | 2.1 | Blend with C2H3CN |
| 503.921239 | C2H3CN | 296,24-295,25 v2 = 1 | 2.0 | 7.9 | 12.5 | |
| 504.297599 | CH3OH | 71-60 | 34.3 | 8.9 | 6.6 | |
| 504.413582 | C2H3CN | 23 6,17/18-23 5,18/19 v2 = 1 | 2.4 | 16.2 | 7.6 | |
| 504.510971 | SO2 | 2310,14-249,15+2310,13-249,16 | 2.4 | 25.3 | 9.5 | |
| 504.680057 | SO | NJ=43-12 | 9.3 | 26.7 | 6.8 | |
| 504.728059 | 34SO | NJ=1211-1110 | 20.5 | 22.4 | 6.6 | Blend with C2H5CN |
| 504.728059 | C2H5CN | 574,54-564,53 | 20.5 | 22.4 | -0.6 | Blend with 34SO |
| 505.431404 | U-line | 2.7 | 7.7 | |||
| 505.499853 | C2H5OH | 296,23+286,22 | 3.5 | 7.9 | 9.3 | |
| 505.765740 | CH3OH | 102-101 | 31.4 | 10.3 | 6.8 | |
| 505.838107 | H2CO | 70,7-60,6 | 47.9 | 17.5 | 6.4 | |
| 506.157737 | CH3OH | 111-102 | 26.0 | 8.3 | 6.3 | |
| 506.240528 | HCOOCH3 | 41 17,25/24-40 17,24/23+41 17,25/24-40 17,23/24 | 13.7 | 25.5 | 6.5 | 4 lines |
| 506.253692 | 34SO | NJ=1212-1111 | 11.0 | 16.9 | 7.6 | |
| 506.773898 | U-line | 6.3 | 10.0 | |||
| 506.831598 | DCN | J=7-6 | 8.7 | 11.8 | 6.6 | |
| 507.041856 | U-line | 4.5 | 5.9 | |||
| 507.200247 | HNCO | 231,22-221,21 | 9.6 | 10.4 | 6.1 | |
| 507.301540 | 34SO | NJ=1213-1112 | 24.9 | 27.8 | 9.0 | |
| 507.337692 | SO2 | 366,30-355,31 | 4.3 | 6.9 | 4.8 | |
| 507.383231 | C2H3CN | 273,24-262,25 v1 = 1 | 8.7 | 6.6 | 6.1 |
![\begin{figure}
\par\includegraphics[width=16.5cm,clip]{h3757fig3.eps}\end{figure}](/articles/aa/full/2003/32/aah3757/Timg93.gif) |
Figure 3: Spectra and lines detected in the survey. The locations of parts of the spectra that were poorly deconvolved are indicated by horizontal lines placed underneath the spectrum. |
![\begin{figure}
\par\includegraphics[width=16cm,clip]{h3757fig4.eps}\end{figure}](/articles/aa/full/2003/32/aah3757/Timg94.gif) |
Figure 4: Spectra and lines detected in the survey. The locations of parts of the spectra that were poorly deconvolved are indicated by horizontal lines placed underneath the spectrum. |
![\begin{figure}
\par\includegraphics[width=16cm,clip]{h3757fig5.eps}\end{figure}](/articles/aa/full/2003/32/aah3757/Timg95.gif) |
Figure 5: Spectra and lines detected in the survey. The locations of parts of the spectra that were poorly deconvolved are indicated by horizontal lines placed underneath the spectrum. |
![\begin{figure}
\par\includegraphics[width=16cm,clip]{h3757fig6.eps}\end{figure}](/articles/aa/full/2003/32/aah3757/Timg96.gif) |
Figure 6: Spectra and lines detected in the survey. The locations of parts of the spectra that were poorly deconvolved are indicated by horizontal lines placed underneath the spectrum. |
In this survey the CO J = 4-3 transition is the most intense single line, with a full width at half maximum of 40 km s-1and full width at zero intensity of at least 120 km s-1. The peak main beam brightness temperature
![]() = 182 K was similar to values that we have previously measured using the JCMT (see for example White & Sandell 1995). The line profile clearly shows a small dip close to its peak,
which is probably a self-absorption dip. This has also been reported by Schilke et al.(2001) in the J = 6-5 transition, and was seen in all of our earlier unpublished JCMT spectra in this transition using both the present SIS receiver, as well as the original JCMT 460-490 GHz Indium Antimonide receiver (White
= 182 K was similar to values that we have previously measured using the JCMT (see for example White & Sandell 1995). The line profile clearly shows a small dip close to its peak,
which is probably a self-absorption dip. This has also been reported by Schilke et al.(2001) in the J = 6-5 transition, and was seen in all of our earlier unpublished JCMT spectra in this transition using both the present SIS receiver, as well as the original JCMT 460-490 GHz Indium Antimonide receiver (White ![]() Padman 1991; Padman
Padman 1991; Padman ![]() White 1992). We have made careful checks on the many occasions
that we have observed this line with the JCMT, confirming that this dip is not a result of the subtraction
of emission located at the off position. Unpublished maps (in preparation) of the spatial
distribution of this absorption feature show it to be spatially localised on the hot core, and that larger scale CO emission is spatially extended around the core - meaning that the
White 1992). We have made careful checks on the many occasions
that we have observed this line with the JCMT, confirming that this dip is not a result of the subtraction
of emission located at the off position. Unpublished maps (in preparation) of the spatial
distribution of this absorption feature show it to be spatially localised on the hot core, and that larger scale CO emission is spatially extended around the core - meaning that the
![]() may overestimate the true kinetic temperature.
may overestimate the true kinetic temperature.
The high abundances of sulphur based molecules in the interstellar medium are believed to be in part due to the presence of shocks, that favour the endothermic reactions required to form these molecules. The rotational temperature diagram for SO2 (see Fig. 7) is consistent with previous estimates of the temperature and
![]()
![]() .
The temperature is 136 K, typical of the cooler conditions found in the plateau. The value of
.
The temperature is 136 K, typical of the cooler conditions found in the plateau. The value of
![]() is estimated to be
is estimated to be
![]() cm-2 in the optically thin limit, increasing to
cm-2 in the optically thin limit, increasing to
![]() cm-2 after making a correction for optical depth. Since our initial assumptions might be that this molecule should almost certainly be moderately optically thick, we ran a model, based on the Sutton et al.(1995) temperature and column density estimate, calculating the expected optical depths for the 35 SO2 lines observed in this survey. One transition has a strong opacity
cm-2 after making a correction for optical depth. Since our initial assumptions might be that this molecule should almost certainly be moderately optically thick, we ran a model, based on the Sutton et al.(1995) temperature and column density estimate, calculating the expected optical depths for the 35 SO2 lines observed in this survey. One transition has a strong opacity
![]() .
The optical depth of all other lines are less than 0.9 and for half of all the detected lines
.
The optical depth of all other lines are less than 0.9 and for half of all the detected lines
![]() .
Therefore the results of the optically thick and optically thin estimates of column density are understandably close to each other. This result is in reasonable agreement with studies using similar beam sizes, by Schilke et al.(2001) (
.
Therefore the results of the optically thick and optically thin estimates of column density are understandably close to each other. This result is in reasonable agreement with studies using similar beam sizes, by Schilke et al.(2001) (
![]() =
=
![]() cm-2 ,
cm-2 , ![]() K for a 12-14
K for a 12-14
![]() beam) and Sutton et al.(1995) (
beam) and Sutton et al.(1995) (
![]() =
=
![]() cm-2 ,
cm-2 , ![]() K for a 12-14
K for a 12-14
![]() beam).
beam).
We further estimate that the isotopic ratio [SO2]/[34SO2] = 14.1, which is in agreement with previous estimates by Blake et al.(1987) (14-16) and Schloerb et al.1983 (11).
The J = 25-24 and J = 27-26 lines of methyl cyanide were observed at 459 and 495 GHz respectively. The average LSR velocity of the lines were ![]() 5.6 km s-1and the line widths
5.6 km s-1and the line widths ![]() 11 km s-1. It is therefore likely that this species traces the hot core. The J = 25-24 k = 7 line (459.276 GHz) could not be assigned due to a strong U-line (459.267 GHz). Lines from the J = 27-26 k = 2
(-1) and k = 3 (+1) transitions were observed at 497.790 and 497.971 GHz respectively, although they were not used in the analysis due to their low intensities. The
11 km s-1. It is therefore likely that this species traces the hot core. The J = 25-24 k = 7 line (459.276 GHz) could not be assigned due to a strong U-line (459.267 GHz). Lines from the J = 27-26 k = 2
(-1) and k = 3 (+1) transitions were observed at 497.790 and 497.971 GHz respectively, although they were not used in the analysis due to their low intensities. The
![]() and
and
![]() values for this molecule were
values for this molecule were
![]() cm-2 and
cm-2 and ![]() 230 K - similar to those estimated by Wilner et al.1994; Sutton et al.1995; Lee et al. (2001). Two lines of the isotopomeric variant CH313CN were also detected. No lines from the v8 = 1 family were identified.
230 K - similar to those estimated by Wilner et al.1994; Sutton et al.1995; Lee et al. (2001). Two lines of the isotopomeric variant CH313CN were also detected. No lines from the v8 = 1 family were identified.
For this molecule, a)
![]() was fixed at 227 K, obtained from analysis of the CH
was fixed at 227 K, obtained from analysis of the CH![]() CN line, and b)
CN line, and b)
![]() and
and
![]() were calculated from the two observed lines. In this latter case, the value of
were calculated from the two observed lines. In this latter case, the value of
![]() is reduced to about one third that of CH
is reduced to about one third that of CH![]() CN. We consider that case a) provides a better solution, because of the difficulty of estimating
CN. We consider that case a) provides a better solution, because of the difficulty of estimating
![]() from two lines whose upper state energy levels are very similar to each other. It did not prove possible to identify the J = 25-24 k = 0, 1, 3 lines due to blending with CH3CN. In view of the problems that have previously been encountered in the interpretation of CH3CN lines using an optically thin assumption
(Schilke et al.1999; Comito et al.2003),
we ran a model to calculate the opacity of the various lines based on the above excitation conditions estimated by Wilner et al.(1994) (obtained with a similar beam size to our study). The largest value of
from two lines whose upper state energy levels are very similar to each other. It did not prove possible to identify the J = 25-24 k = 0, 1, 3 lines due to blending with CH3CN. In view of the problems that have previously been encountered in the interpretation of CH3CN lines using an optically thin assumption
(Schilke et al.1999; Comito et al.2003),
we ran a model to calculate the opacity of the various lines based on the above excitation conditions estimated by Wilner et al.(1994) (obtained with a similar beam size to our study). The largest value of ![]() amongst the present lines is estimated to be 0.2, and all other values were
amongst the present lines is estimated to be 0.2, and all other values were ![]() 0.1. Making a correction for the optical depth only increases the value of column density by a few percent - from
0.1. Making a correction for the optical depth only increases the value of column density by a few percent - from
![]() =
=
![]() cm-2 to
cm-2 to
![]() cm-2 . We therefore conclude that with few exceptions, the optically thin assumption provides a valid and useful estimate of column density for the transitions observed in this survey.
cm-2 . We therefore conclude that with few exceptions, the optically thin assumption provides a valid and useful estimate of column density for the transitions observed in this survey.
Thirteen lines of vinyl cyanide and its isotopomeric variant C![]() H
H![]() CN were detected at LSR velocities consistent with the molecules being concentrated in the compact ridge. Line frequencies have been reported by Demaison et al.(1994). 5 lines were tentatively associated with the v15 (out-of-plane bend) = 1 vibrational excited state. However, since the frequencies of the v15 lines have a large error, they were not included in the fitting. We also searched at the expected frequency of the
v11 = 2 state, but could not find convincing match. Grain surface reactions are thought to be the main process by which complex nitrogen bearing species are formed, due the high level of hydrogenation. It is not likely that a molecule such as CCCN would pick up the required number of hydrogen atoms to become ethyl cyanide without the intermediate step of adsorption on to a grain, allowing the hydrogenation process to occur. The high temperatures found in the hot core are sufficient to evaporate the molecules from the surface of any grains that drift into the region.
CN were detected at LSR velocities consistent with the molecules being concentrated in the compact ridge. Line frequencies have been reported by Demaison et al.(1994). 5 lines were tentatively associated with the v15 (out-of-plane bend) = 1 vibrational excited state. However, since the frequencies of the v15 lines have a large error, they were not included in the fitting. We also searched at the expected frequency of the
v11 = 2 state, but could not find convincing match. Grain surface reactions are thought to be the main process by which complex nitrogen bearing species are formed, due the high level of hydrogenation. It is not likely that a molecule such as CCCN would pick up the required number of hydrogen atoms to become ethyl cyanide without the intermediate step of adsorption on to a grain, allowing the hydrogenation process to occur. The high temperatures found in the hot core are sufficient to evaporate the molecules from the surface of any grains that drift into the region.
Twenty seven lines of ethyl cyanide were detected, based on line frequencies taken from Pearson et al.(1997) and Pearson (2000). It is expected that many observed lines of this molecule are
blended with U-lines, and it was found that the simultaneous estimation of the column density and rotational temperature were difficult, since many of the lines are located very close together on the rotational diagram. To avoid this problem. we have calculated the column densities of C![]() H
H![]() CN for two fixed temperatures, deriving values of
CN for two fixed temperatures, deriving values of
![]() =
=
![]() cm-2 (assuming a temperature of
150 K based on the work of Sutton et al.(1985), or
cm-2 (assuming a temperature of
150 K based on the work of Sutton et al.(1985), or
![]() =
=
![]() cm-2 (assuming a temperature of 239 K based on the work of Schilke et al.(2001). As a result, the best estimates of the column density of the C
cm-2 (assuming a temperature of 239 K based on the work of Schilke et al.(2001). As a result, the best estimates of the column density of the C![]() H
H![]() CN from the present data are in the range
CN from the present data are in the range
![]() = 2.4-
= 2.4-
![]() cm-2 . This value is close to that estimated by Schilke et al.(2001) (
cm-2 . This value is close to that estimated by Schilke et al.(2001) (
![]() =
=
![]() cm-2 ) with a beam size of 10-12
cm-2 ) with a beam size of 10-12
![]() - which is very similar to that of the present survey. By comparison, Sutton et al.(1985) reported
- which is very similar to that of the present survey. By comparison, Sutton et al.(1985) reported
![]() =
=
![]() cm-2 with a 30
cm-2 with a 30
![]() beam.
Assuming that the emitting region is a core with 10
beam.
Assuming that the emitting region is a core with 10
![]() diameter,
diameter,
![]() =
=
![]() cm-2 , which is in better agreement with the estimate in the present survey value. Similar arguments applied to the methyl cyanide line, to resolve the differences between the column densities from this survey, and the work of Blake et al.(1985) and Schilke et al.(2001), again suggest that the size of the emitting region is
cm-2 , which is in better agreement with the estimate in the present survey value. Similar arguments applied to the methyl cyanide line, to resolve the differences between the column densities from this survey, and the work of Blake et al.(1985) and Schilke et al.(2001), again suggest that the size of the emitting region is ![]() 10
10
![]() .
.
Using only two lines, it did not prove possible to get a reasonable value for
![]() .
Hence, in fitting the value of
.
Hence, in fitting the value of
![]() was fixed at 150 K, from observations of HNCO, which has a similar dipole moment (CH
was fixed at 150 K, from observations of HNCO, which has a similar dipole moment (CH![]() NH
NH
![]() = 1.352 D,
= 1.352 D,
![]() = 1.530 D,
HNCO
= 1.530 D,
HNCO
![]() = 1.602 D,
= 1.602 D,
![]() = 1.35 D -
from Kirchoff et al. 1973; Hocking et al.1975). The derived column density,
= 1.35 D -
from Kirchoff et al. 1973; Hocking et al.1975). The derived column density,
![]() =
=
![]() cm-2 , is in reasonable agreement with the column density reported in the detection paper by Dickens et al.(1977) of
cm-2 , is in reasonable agreement with the column density reported in the detection paper by Dickens et al.(1977) of
![]() =
=
![]() cm-2 , based on observations of 5 transitions (three of which were blended with other lines) between 172 and 256 GHz obtained with a larger beam of 23-34
cm-2 , based on observations of 5 transitions (three of which were blended with other lines) between 172 and 256 GHz obtained with a larger beam of 23-34
![]() .
.
Methanol, CH![]() OH, is one of the most widely observed molecules in star forming regions. Line frequencies are reported by Xu
OH, is one of the most widely observed molecules in star forming regions. Line frequencies are reported by Xu ![]() Lovas (1997). Twenty four CH
Lovas (1997). Twenty four CH![]() OH lines were detected with an average velocity of 7.8 km s-1 and line width of 9.9 km s-1 - suggesting that it is likely to be excited in the compact ridge. The high observed abundances of this molecule imply a high abundance of the precursor ion CH
OH lines were detected with an average velocity of 7.8 km s-1 and line width of 9.9 km s-1 - suggesting that it is likely to be excited in the compact ridge. The high observed abundances of this molecule imply a high abundance of the precursor ion CH
![]() which would react with water in a ion-molecule process to form methanol. Two lines of
which would react with water in a ion-molecule process to form methanol. Two lines of ![]() CH
CH![]() OH (which were apparently detected at 492.695 and 498.677 GHz) would indicate that the main methanol line may have an opacity of at least 0.7. Schilke et al.(2001) have pointed out the difficulty of applying simple rotational analysis techniques to methanol due to opacity problems. The column densities reported in Table 1 have been should therefore be treated as lower limits. If the 498.677 GHz line is assigned to
OH (which were apparently detected at 492.695 and 498.677 GHz) would indicate that the main methanol line may have an opacity of at least 0.7. Schilke et al.(2001) have pointed out the difficulty of applying simple rotational analysis techniques to methanol due to opacity problems. The column densities reported in Table 1 have been should therefore be treated as lower limits. If the 498.677 GHz line is assigned to ![]() CH
CH![]() OH-A(82-81), the column density of this molecule would be
OH-A(82-81), the column density of this molecule would be
![]() cm-2 assuming the rotational temperature is the same as that of
cm-2 assuming the rotational temperature is the same as that of ![]() CH
CH![]() OH (600 K - but note that the error on this is 50%). This would indicate that the [12C]/[13C] ratio
OH (600 K - but note that the error on this is 50%). This would indicate that the [12C]/[13C] ratio ![]() 10 - although this would also decrease if the temperature were substantially less than 600K). Neither the
10 - although this would also decrease if the temperature were substantially less than 600K). Neither the ![]() CH
CH![]() OH-E(23-2-23-1) line at 503.216 GHz, nor any lines of torsionally excited methanol were identified.
OH-E(23-2-23-1) line at 503.216 GHz, nor any lines of torsionally excited methanol were identified.
Eight lines of C![]() H
H![]() OH were detected, at a velocity of
OH were detected, at a velocity of ![]() 8 km s-1, although only 6 were used in the rotational temperature fitting. Line frequencies are given by Pearson et al.(1996). The lines at 494.899 and 495.821 had line widths that were too broad and narrow respectively, and it is likely that these are blended, or due to other species. For this molecule, we made two fits; a) simultaneously fitting both
8 km s-1, although only 6 were used in the rotational temperature fitting. Line frequencies are given by Pearson et al.(1996). The lines at 494.899 and 495.821 had line widths that were too broad and narrow respectively, and it is likely that these are blended, or due to other species. For this molecule, we made two fits; a) simultaneously fitting both
![]() and
and
![]() ,
and b)
,
and b)
![]() was fixed at 70 K (following Ohishi et al.1995), since simultaneous fitting of both
was fixed at 70 K (following Ohishi et al.1995), since simultaneous fitting of both
![]() and
and
![]() led to large uncertainties, and a large
led to large uncertainties, and a large
![]() .
.
Dimethyl Ether, (CH![]() )
)![]() O, is one of the few interstellar molecules whose emission lines are affected by the presence of two internal rotors. Line frequencies are reported by Groner et al.(1998). Twenty seven lines were detected in this survey, which were best described by a fit of
O, is one of the few interstellar molecules whose emission lines are affected by the presence of two internal rotors. Line frequencies are reported by Groner et al.(1998). Twenty seven lines were detected in this survey, which were best described by a fit of
![]() =
=
![]() cm-2 and
cm-2 and
![]() = 157 K for the column density and rotational temperature respectively. These values are close to those found by Sutton et al (1985) toward the compact ridge. The line widths and velocities are consistent with an origin in the compact ridge. The fact that this molecule appears to be far more abundant (from the large number of strong lines) than ethanol leads to the conclusion that the Compact ridge, where these molecules are formed, is rich in hydrogen. The molecule is formed by a similar method to methyl formate except that CH
= 157 K for the column density and rotational temperature respectively. These values are close to those found by Sutton et al (1985) toward the compact ridge. The line widths and velocities are consistent with an origin in the compact ridge. The fact that this molecule appears to be far more abundant (from the large number of strong lines) than ethanol leads to the conclusion that the Compact ridge, where these molecules are formed, is rich in hydrogen. The molecule is formed by a similar method to methyl formate except that CH![]() OH
OH
![]() reacts with methanol to form it.
reacts with methanol to form it.
A single transition of HDO was detected at 464.925 GHz. It has a clear hot
core line shape with a line width of 16.2 km s-1 and a velocity of 6.6 km s-1. We have attempted to model this line along with the two other lines reported and analysed by Schulz et al.(1991) and Sutton et al.(1985). Pardo et al.(2001b) have recently reported detections of the
![]() -1
-1
![]() and 1
and 1
![]() -
-![]() lines in the 850-900 GHz region, which appear to trace the plateau gas, rather than the hot core material which contributes rather more to the other HDO lines observed to date. We therefore estimate values of
lines in the 850-900 GHz region, which appear to trace the plateau gas, rather than the hot core material which contributes rather more to the other HDO lines observed to date. We therefore estimate values of
![]() cm-2 and 164 K for
cm-2 and 164 K for
![]() and
and
![]() respectively, in broad agreement with values reported by Plambeck & Wright (1987) and Jacq et al.(1990). This is as expected from the high abundances observed for molecules such as methanol and methyl formate which require water to be present for their formation. It is thought that both water and deuterated water are formed elsewhere on grains and evaporated from the surfaces in the higher temperature conditions found in the hot core (Beckman et al.1981; Pardo et al.2001b).
respectively, in broad agreement with values reported by Plambeck & Wright (1987) and Jacq et al.(1990). This is as expected from the high abundances observed for molecules such as methanol and methyl formate which require water to be present for their formation. It is thought that both water and deuterated water are formed elsewhere on grains and evaporated from the surfaces in the higher temperature conditions found in the hot core (Beckman et al.1981; Pardo et al.2001b).
At total of 33 lines were detected that could not be associated with known spectral lines. We searched carefully in the Cologne Database for Molecular Spectroscopy (Müller et al.2001) and the JPL Molecular Spectroscopy Database, as well as other tables of isotopomeric variants of lines that were present in the survey (e.g. CH![]() OH and
OH and ![]() CH
CH![]() OH from the laboratory measurements of Anderson et al. 1987, 1990, 1992). Although there were inevitably a number of lines that lay
close to some of the U-line frequencies, we also used secondary criteria (line strength predictions from the Cologne database, upper energy state levels, and the presence or absence of other lines from similar levels) to make judgments as to whether lines could be associated with particular species. Six lines that were originally designated as U-lines were associated in this way, however a substantial number of intense lines remain. Assignment of lines to these frequencies is beyond the scope of this paper, and will require a sophisticated modeling effort, combined with U-lines from other published surveys. We note that the number of U-lines inferred from the present survey (33 - or 13% of the total) is similar reported from some other surveys (325-360 GHz
OH from the laboratory measurements of Anderson et al. 1987, 1990, 1992). Although there were inevitably a number of lines that lay
close to some of the U-line frequencies, we also used secondary criteria (line strength predictions from the Cologne database, upper energy state levels, and the presence or absence of other lines from similar levels) to make judgments as to whether lines could be associated with particular species. Six lines that were originally designated as U-lines were associated in this way, however a substantial number of intense lines remain. Assignment of lines to these frequencies is beyond the scope of this paper, and will require a sophisticated modeling effort, combined with U-lines from other published surveys. We note that the number of U-lines inferred from the present survey (33 - or 13% of the total) is similar reported from some other surveys (325-360 GHz ![]() 8% Sutton et al.1995), 607-725 GHz
8% Sutton et al.1995), 607-725 GHz ![]() 14% (Comito et al.2003).
14% (Comito et al.2003).
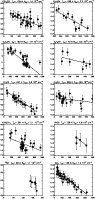 |
Figure 7: Rotational temperature diagrams. The fitted lines were derived from a noise-weighted least squares fitting procedure. |
The values for the intrinsic line strengths, level degeneracies and partition functions are derived from the literature, including the spectral line catalogues cited previously (see also Eqs. (1) and (2)).
Acknowledgements
We acknowledge discussions with Prof S. Saito at Fukui University, Dr Y. Fukuyama at the Institute of Physical and Chemical Research, Prof John Pearson, for discussions about frequency assignments of molecules, and the referee for helpful comments.